Abstract
Bone-resorbing osteoclasts significantly contribute to osteoporosis and bone metastases of cancer1,2,3. MicroRNAs play important roles in physiology and disease4,5, and present tremendous therapeutic potential6. Nonetheless, how microRNAs regulate skeletal biology is underexplored. Here we identify miR-34a as a novel and critical suppressor of osteoclastogenesis, bone resorption and the bone metastatic niche. miR-34a is downregulated during osteoclast differentiation. Osteoclastic miR-34a-overexpressing transgenic mice exhibit lower bone resorption and higher bone mass. Conversely, miR-34a knockout and heterozygous mice exhibit elevated bone resorption and reduced bone mass. Consequently, ovariectomy-induced osteoporosis, as well as bone metastasis of breast and skin cancers, are diminished in osteoclastic miR-34a transgenic mice, and can be effectively attenuated by miR-34a nanoparticle treatment. Mechanistically, we identify transforming growth factor-β-induced factor 2 (Tgif2) as an essential direct miR-34a target that is pro-osteoclastogenic. Tgif2 deletion reduces bone resorption and abolishes miR-34a regulation. Together, using mouse genetic, pharmacological and disease models, we reveal miR-34a as a key osteoclast suppressor and a potential therapeutic strategy to confer skeletal protection and ameliorate bone metastasis of cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
01 June 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41586-020-2273-1
24 May 2019
A Correction to this paper has been published: https://doi.org/10.1038/s41586-019-1266-4
References
Coleman, R. E. Bone cancer in 2011: prevention and treatment of bone metastases. Nature Rev. Clin. Oncol. 9, 76–78 (2012)
Ell, B. & Kang, Y. SnapShot: bone metastasis. Cell 151, 690 (2012)
Novack, D. V. & Teitelbaum, S. L. The osteoclast: friend or foe? Annu. Rev. Pathol. 3, 457–484 (2008)
Chivukula, R. R. & Mendell, J. T. Circular reasoning: microRNAs and cell-cycle control. Trends Biochem. Sci. 33, 474–481 (2008)
Ventura, A. & Jacks, T. MicroRNAs and cancer: short RNAs go a long way. Cell 136, 586–591 (2009)
Kasinski, A. L. & Slack, F. J. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nature Rev. Cancer 11, 849–864 (2011)
Wan, Y., Chong, L. W. & Evans, R. M. PPAR-γ regulates osteoclastogenesis in mice. Nature Med. 13, 1496–1503 (2007)
Wei, W. et al. PGC1β mediates PPARγ activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 11, 503–516 (2010)
Choi, Y. J. et al. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nature Cell Biol. 13, 1353–1360 (2011)
Concepcion, C. P. et al. Intact p53-dependent responses in miR-34-deficient mice. PLoS Genet. 8, e1002797 (2012)
Bae, Y. et al. miRNA-34c regulates Notch signaling during bone development. Hum. Mol. Genet. 21, 2991–3000 (2012)
Wei, J. et al. miR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J. Cell Biol. 197, 509–521 (2012)
Ell, B. et al. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 24, 542–556 (2013)
Yamakuchi, M., Ferlito, M. & Lowenstein, C. J. miR-34a repression of SIRT1 regulates apoptosis. Proc. Natl Acad. Sci. USA 105, 13421–13426 (2008)
Lefort, K. et al. A miR-34a-SIRT6 axis in the squamous cell differentiation network. EMBO J. 32, 2248–2263 (2013)
Boon, R. A. et al. MicroRNA-34a regulates cardiac ageing and function. Nature 495, 107–110 (2013)
Lodygin, D. et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle 7, 2591–2600 (2008)
Hermeking, H. p53 enters the microRNA world. Cancer Cell 12, 414–418 (2007)
Liu, C. et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nature Med. 17, 211–215 (2011)
Fukuda, T. et al. Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2. Genesis 44, 159–167 (2006)
Wei, W. et al. Osteoclast progenitors reside in the peroxisome proliferator-activated receptor gamma-expressing bone marrow cell population. Mol. Cell. Biol. 31, 4692–4705 (2011)
Clausen, B. E., Burkhardt, C., Reith, W., Renkawitz, R. & Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999)
Nakamura, T. et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130, 811–823 (2007)
Maes, C. et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329–344 (2010)
Powers, S. E. et al. Tgif1 and Tgif2 regulate Nodal signaling and are required for gastrulation. Development 137, 249–259 (2010)
Lu, C. et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell 18, 185–197 (2010)
Wei, W. et al. Biphasic and dosage-dependent regulation of osteoclastogenesis by β-catenin. Mol. Cell. Biol. 31, 4706–4719 (2011)
Lu, X. et al. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev. 23, 1882–1894 (2009)
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003)
Uluckan, O. et al. APT102, a novel adpase, cooperates with aspirin to disrupt bone metastasis in mice. J. Cell. Biochem. 104, 1311–1323 (2008)
Chang, T. C. et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nature Genet. 40, 43–50 (2008)
Acknowledgements
We thank University of Texas Southwestern transgenic core and small animal imaging core for their assistance in our studies; P. Dechow, J. Feng and C. Qin for assistance with micro-computed tomography, histomorphometry and X-ray analysis; A. Ventura for miR-34abc triple knockout mice; D. Wotton for Tgif2-KO mice; H. Kronenberg for Osx-CreER mice; Y. Mishina for CAG-Z-EGFP vector. Y. Wan is a Virginia Murchison Linthicum Scholar in Medical Research. This work was in part supported by CPRIT (RP130145, Y.W.; R1008, J.M.), DOD (BC122877, Y.W.), National Institutes of Health (R01 DK089113, Y.W.; R01 CA120185 and P01 CA134292, J.M.; U54 CA151668 and UH2 TR000943, A.S.; R01 CA139067, L.H.), The Welch Foundation (I-1751, Y.W.) and a University of Texas Southwestern Endowed Scholar Startup Fund (Y.W.). The University of Texas Southwestern Small Animal Imaging Resource is supported in part by the Harold C. Simmons Cancer Center through an NCI Cancer Center Support Grant (1P30 CA142543) and The Department of Radiology. The VisualSonics Vevo 770 was purchased with National Institutes of Health American Recovery and Reinvestment Act stimulus funds 1S10RR02564801.
Author information
Authors and Affiliations
Contributions
J.Y.K. and Y.W. conceived the project and designed the experiments. All experiments, except those listed below, were performed by J.Y.K. W.W. assisted with micro-computed tomography, enzyme-linked immunosorbent assay and histomorphometry analyses. H.D.H. assisted with bone marrow transplantation and injection. Z.J. assisted with FACS analyses. X.W. assisted with western blot analyses. T.C.C. assisted with northern blot analyses and lifespan experiments. X.J.X. assisted with statistical analyses. L.H. provided the full miR-34a knockout mice. L.S.M., G.L.B. and A.K.S. assisted with nanoparticle packaging. J.T.M. provided the miR-34a gene trap knockout mice. Y.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
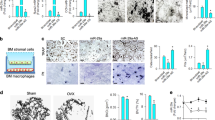
Extended Data Figure 1 Additional analyses of 34a-Tie2-Tg mice.
a–c, Further characterization of the transgene expression in 34a-Tie2-Tg mice. a, FACS analysis of the percentage of GFP+ bone marrow osteoclast progenitors (c-Fms+RANK+) in 34a-Tie2-Tg mice and ‘transgene only, no cre’ control (n = 3). b, GFP and LacZ expression in osteoclast progenitors from 34a-Tie2-Tg mice (GFP+LacZ−) and ‘transgene only, no cre’ control mice (GFP−LacZ+). Scale bar, 100 μm. c, Northern blot analysis confirmed miR-34a overexpression in the haematopoietic bone marrow cells of 34a-Tie2-Tg mice. Ct, control; Tg, 34a-Tie2-Tg; EtBr, ethidium bromide. d, Quantitative PCR of mRNA expression of additional osteoclast marker genes (n = 3). e, Osteoclast function analysis. Bone marrow osteoclast differentiation was conducted in OsteoAssay bone plates (Lonza), and osteoclast activity was quantified as calcium release using CalciFluo ELISA assay (Lonza) (n = 8, mean ± s.e.m.). f, Osteoclast proliferation was not affected, quantified by BrdU incorporation (n = 6). g, Osteoclast apoptosis was not affected, quantified by FACS analysis of AnnexinV+7-AAD− cells (n = 6). h, i, Static and dynamic histomorphometry. h, Representative images of distal femur sections (2-month-old, male). Scale bars, 1 mm for Von Kossa images; 10 μm for TRAP, ALP and calcein images. i, Quantification of parameters at distal femur and vertebrae in 2-month-old male and female mice. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001; n.s., non-significant.
Extended Data Figure 2 Effects of miR-34a overexpression using additional cre driver targeting osteoclast progenitors.
34a-PT-Tg mice were generated using PPAR-γ-tTA-TRE-cre driver. a, Bone marrow osteoclast differentiation assays. Left, mature miR-34a level (n = 3); middle, TRAP mRNA expression (n = 3); right, TRAP staining of differentiation cultures, quantification of mature osteoclast numbers per well in 24-well plates (black, n = 3), and quantification of bone resorptive activity by calcium release from bone plate into culture medium (μM) (blue, n = 6). b, Serum CTX-1 bone resorption marker (2-month-old males, n = 10). c, Micro-computed tomography analysis of the trabecular bone in proximal tibiae (2-month-old males, n = 4). d, Histomorphometry of the distal femur and vertebrae in 2-month-old mice. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
Extended Data Figure 3 Effects of miR-34a overexpression using additional osteoclastic cre drivers.
a–d, 34a-Lys-Tg mice were generated using Lysozyme-cre driver. e–h, 34a-Ctsk-Tg mice were generated using Ctsk-cre driver. a, e, Bone marrow osteoclast differentiation assays. Left, mature miR-34a level (n = 3); middle, TRAP mRNA expression (n = 3); right, TRAP staining of differentiation cultures, quantification of mature osteoclast numbers per well in 24-well plates (black, n = 3), and quantification of bone resorptive activity by calcium release from bone plate into culture medium (μM) (blue, n = 6). b, f, Serum CTX-1 (2-month-old males; b, n = 5; f, n = 8). c, g, Trabecular BV/TV of proximal tibiae by micro-computed tomography (2-month-old males; c, n = 4; g, n = 4). d, h, Histomorphometry of the distal femur and vertebrae in 2-month-old mice. *P < 0.05, **P < 0.01, ***P < 0.005.
Extended Data Figure 4 Additional analyses of gene-trap miR-34a knockout mice.
a, Northern blot analysis confirmed decreased miR-34a expression in the miR-34a gene trap knockout mice. Six-week-old female mice with corresponding genotypes were irradiated with a dose of 6 Gy, and 4 h later the spleen was collected for RNA extraction. Northern blotting for miR-34a was performed as described31. b, Quantitative PCR of mRNA expression of additional osteoclast marker genes (n = 3). c, Osteoclast function analysis. Bone marrow osteoclast differentiation was conducted in OsteoAssay bone plates (Lonza), and osteoclast activity was quantified as calcium release using CalciFluo ELISA assay (Lonza) (n = 8, mean ± s.e.m.). d, Osteoclast proliferation was not affected, quantified by BrdU incorporation (n = 6). e, Osteoclast apoptosis was not affected, quantified by FACS analysis of AnnexinV+7-AAD− cells (n = 6). f, WT mice transplanted with 34a-KO bone marrow cells exhibited higher serum CTX-1 levels than WT mice transplanted with WT bone marrow cells (n = 5 recipients per group). g, h, Static and dynamic histomorphometry. g, Representative images of distal femur sections (2-month-old, male). Scale bars, 1 mm for Von Kossa images; 10 μm for TRAP, ALP and Calcein images. h, Quantification of parameters at distal femur and vertebrae in 2-month-old male and female mice. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001; n.s., non-significant.
Extended Data Figure 5 Effects of targeted miR-34a deletion.
a–d, Targeted miR-34a/b/c triple knockout (34abc-TKO) mice were compared with WT control mice (5-month-old males, n = 4). a–c, Bone marrow osteoclast differentiation assay. a, Expression of miR-34a was diminished whereas expression of miR-34b and miR-34c remained absent/low in osteoclast precursors on d3. b, Expression of osteoclast markers was increased. c, Number, size and resorptive activity of mature osteoclasts were increased. d, Serum CTX-1 was increased. e–h, Targeted full miR-34a knockout (34a-full-KO) mice were compared with WT control mice (2-month-old females, n = 3). e–g, Bone marrow osteoclast differentiation assay. e, Expression of miR-34a was diminished in osteoclast precursors on day 3. f, Expression of osteoclast markers was increased. g, Number, size and resorptive activity of mature osteoclasts were increased. h, Serum CTX-1 was increased. i–n, Conditional miR-34a knockout mice by Tie2-cre (34a-Tie2-KO) were compared with littermate miR-34af/f control mice (2-month-old males, n = 6). i–k, Bone marrow osteoclast differentiation assay. i, miR-34a expression was reduced in osteoclast precursors on day 3. j, Expression of osteoclast markers was increased. k, Number, size and resorptive activity of mature osteoclasts were increased. l, Serum CTX-1 was increased. m, Trabecular BV/TV of proximal tibiae by micro-computed tomography. n, Histomorphometry of the distal femur and vertebrae. For c, g, k, mature osteoclasts were identified as multinucleated (more than three nuclei) TRAP+ (purple) cells. Scale bar, 25 μm. Quantification of osteoclast number/well is shown in black. Quantification of osteoclast resorptive activity by calcium release from bone to culture medium (μM) is shown in blue. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
Extended Data Figure 6 Anti-osteoporosis effects of miR-34a.
a, Histomorphometry of the distal femur and vertebrae in OVX mice treated with miR-34a-CH nanoparticles. OVX or sham operation was performed on 10-week-old WT female C57BL/6J mice. Three days after surgery, the OVX mice were intravenously injected with miR-34a-CH (34a) or miR-Ctrl-CH (Ctrl) at 5 μg per mouse twice a week for 5 weeks (n = 5). b–d, Osteoprotective effects of miR-34a-CH in sham control mice. WT female C57B/6J mice (n = 5, 10 weeks old) were subjected to sham operation and then treated with miR-34a-CH or miR-34a-Ctrl at 5 μg per mouse twice a week for 5 weeks. b, Serum CTX-1. c, Serum P1NP. d, BV/TV of proximal tibiae by micro-computed tomography. e, Histomorphometry of the distal femur and vertebrae in WT and 34a-Tie2-Tg mice after OVX. 34a-Tie2-Tg mice or controls (3-month-old females, n = 7) were subjected to OVX or sham operation and analysed 5 weeks after surgery. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
Extended Data Figure 7 Additional characterization of bone metastases.
a, Representative BLI images. b, Quantification of the number of metastasis. c, Quantification of the size of metastasis. For a–c, n = 9 for control, n = 8 for 34a-Tie2-Tg, n = 6 for WT and 34a-KO; results are shown as mean ± s.e.m. d, Xenograft of MDA231-BoM-1833 human breast cancer cells into 34a-Tie2-Tg nude mice (n = 8) or littermate control nude mice (n = 9). Results from each week are shown separately to visualize the difference better. e, Xenograft of MDA231-BoM-1833 human breast cancer cells into 34a-PT-Tg nude mice (n = 8) or littermate control nude mice (n = 8). Results from each week are shown separately to visualize the difference better. f, Allograft of B16-F10 mouse melanoma cells into 34a-PT-Tg (n = 7) or littermate control mice (n = 7). *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001; n.s., non-significant.
Extended Data Figure 8 Effects of miR-34a on cancer cells.
a, Systemic miR-34a-CH delivery did not affect the growth of B16-F10 melanoma cells injected subcutaneously (n = 5, 8-week-old males). Tumours were collected 18 days after cell injection; the result is shown as mean ± s.e.m. b, Systemic miR-34a-CH delivery did not affect cancer metastasis to other organs such as lung (n = 5, 8-week-old males). B16-F10 cells were intravenously injected retro-orbitally, BLI signals were quantified 2 weeks later and the result is shown as mean ± s.e.m. c, d, MiR-34a-CH treatment of cancer cell alone was not sufficient to inhibit bone metastasis. BoM-1833 cells were treated with miR-34a-CH or miR-Ctrl-CH in cultures for 24 h before cardiac injection (n = 5, 6-week-old males), and the mice were not treated with nanoparticles. c, Quantification of bone metastasis BLI signal 5 weeks after injection, shown as mean ± s.e.m. d, MiR-34a overexpression in BoM-1833 cells persisted for 5 weeks in cultures. e, Loss-of-function in 34a-KO and 34a-Het mice did not result in significantly increased susceptibility of cancer and mortality. Left, Kaplan–Meier survival curve for WT (n = 29), 34a-Het (n = 35) and 34a-KO (n = 29); P = 0.223 by log-rank (Mantel–Cox) test. Right, the 34a-KO allele was transmitted at normal Mendelian frequency. ****P < 0.001; n.s., non-significant.
Extended Data Figure 9 Osteoblastic miR-34a overexpression is not sufficient to inhibit osteoporosis or bone metastases.
a, Schematic diagram of the ex vivo bone marrow osteoblast differentiation assay. MSC GF, mesenchymal stem cell growth factors; GP, β-glycerophosphate; AA, ascorbic acid. b, Osteoblast differentiation was decreased for bone marrow from 34a-KO and 34a-Het mice compared with WT controls, quantified by osteoblast marker genes osteocalcin and Col1a1 on day 13 (n = 6). c–h, Characterization of osteoblastic miR-34a transgenic mice. CAG34a mice were bred with Osterix-CreER mice to generate miR34a-Osx-transgenic (34a-Osx-Tg) mice or littermate control mice that carry only CAG34a transgene; all mice (1-month-old, male) received tamoxifen injection on two consecutive days and analysed 2 months later. c, Elevated levels of mature miR-34a in 34a-Osx-Tg osteoblast differentiation cultures on day 13 (n = 6). d, Osteoblast differentiation was increased for bone marrow from 34a-Osx-Tg mice compared with control mice, quantified by osteoblast marker genes osteocalcin and Col1a1 on day 13 (n = 6). e, Serum P1NP was increased in 34a-Osx-Tg mice (n = 6). f, Serum CTX-1 was unaltered in 34a-Osx-Tg mice (n = 6). g, Histomorphometry of distal femur and vertebrae in 34a-Osx-Tg and control mice. h, OVX-induced bone resorption and bone loss was unaltered in 34a-Osx-Tg mice. 34a-Osx-Tg mice or controls (3-month-old females, 2 months after tamoxifen injection, n = 5) were subjected to OVX or sham operation and analysed 5 weeks after surgery. i, Cancer bone metastasis was unaltered in 34a-Osx-Tg mice (n = 8). Statistical analyses in i were performed with a Mann–Whitney U Test and are shown as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001; n.s., non-significant.
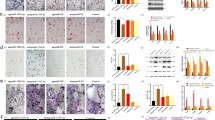
Extended Data Figure 10 Additional characterization of Tgif2 as a key miR-34a direct target gene.
a, A list of potential miR-34a target genes in the osteoclast lineage and characterization of miR-34a regulation. N.D., not determined. b, Fold changes in the expression of each candidate target gene after transfection with pre-miR-34a compared with pre-miR-ctrl in WT bone marrow osteoclast differentiation culture (n = 3). c, Fold changes in the luciferase readout from 3′ UTR reporter for each candidate target gene co-transfected in HEK293 cells with pre-miR-34a or pre-control. The results were normalized by internal control β-galactosidase (β-gal) readout (n = 3). d, Western blot analysis showing that Tgif2 protein expression is decreased in the bone marrow osteoclast progenitors from 34a-Tie2-Tg transgenic mice compared with control mice (left), but increased in the bone marrow osteoclast progenitors from 34a-KO and 34a-Het mice compared with WT control mice (right). e, Human Tgif2 expression in hPBMN osteoclast differentiation cultures was suppressed by pre-miR-34a but enhanced by anti-miR-34a via transfection (n = 4). f, Histomorphometry of the distal femur and vertebrae in 1.5-month-old Tgif2-KO, Tgif2-Het and WT control mice. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001; n.s., non-significant.
About this article
Cite this article
Krzeszinski, J., Wei, W., Huynh, H. et al. RETRACTED ARTICLE: miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature 512, 431–435 (2014). https://doi.org/10.1038/nature13375
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13375
This article is cited by
-
Phosphorylation of TGIF2 represents a therapeutic target that drives EMT and metastasis of lung adenocarcinoma
BMC Cancer (2023)
-
Circular RNA circStag1 promotes bone regeneration by interacting with HuR
Bone Research (2022)
-
Serum lnc34a is a potential prediction biomarker for bone metastasis in hepatocellular carcinoma patients
BMC Cancer (2021)
-
Silencing long noncoding RNA colon cancer-associated transcript-1 upregulates microRNA-34a-5p to promote proliferation and differentiation of osteoblasts in osteoporosis
Cancer Gene Therapy (2021)
-
N6-methyladenosine demethylase ALKBH5 suppresses malignancy of esophageal cancer by regulating microRNA biogenesis and RAI1 expression
Oncogene (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.