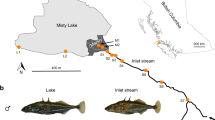
Abstract
Ecological differences often evolve early in speciation as divergent natural selection drives adaptation to distinct ecological niches, leading ultimately to reproductive isolation. Although this process is a major generator of biodiversity, its genetic basis is still poorly understood. Here we investigate the genetic architecture of niche differentiation in a sympatric species pair of threespine stickleback fish by mapping the environment-dependent effects of phenotypic traits on hybrid feeding and performance under semi-natural conditions. We show that multiple, unlinked loci act largely additively to determine position along the major niche axis separating these recently diverged species. We also find that functional mismatch between phenotypic traits reduces the growth of some stickleback hybrids beyond that expected from an intermediate phenotype, suggesting a role for epistasis between the underlying genes. This functional mismatch might lead to hybrid incompatibilities that are analogous to those underlying intrinsic reproductive isolation but depend on the ecological context.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Darwin, C. The Origin of Species by Means of Natural Selection (John Murray, 1859)
Schluter, D. The Ecology of Adaptive Radiation (Oxford Univ. Press, 2000)
Coyne, J. A. & Orr, H. A. Speciation (Sinauer Associates, 2004)
Nosil, P. Ecological Speciation (Oxford Univ. Press, 2012)
Chase, J. M. & Leibold, M. A. Ecological Niches: Linking Classical and Contemporary Approaches (Univ. of Chicago Press, 2003)
Fisher, R. A. The Genetical Theory of Natural Selection (Oxford Univ. Press, 1930)
Orr, H. A. The genetic theory of adaptation: a brief history. Nature Rev. Genet. 6, 119–127 (2005)
Gavrilets, S. Fitness Landscapes and the Origin of Species (Monographs in Population Biology Vol. 41, Princeton Univ. Press, 2004)
Yeaman, S. & Whitlock, M. C. The genetic architecture of adaptation under migration-selection balance. Evolution 65, 1897–1911 (2011)
Mackay, T. F. C., Stone, E. A. & Ayroles, J. F. The genetics of quantitative traits: challenges and prospects. Nature Rev. Genet. 10, 565–577 (2009)
Barrett, R. D. H. & Hoekstra, H. E. Molecular spandrels: tests of adaptation at the genetic level. Nature Rev. Genet. 12, 767–780 (2011)
Taylor, E. B. & McPhail, J. D. Historical contingency and ecological determinism interact to prime speciation in sticklebacks, Gasterosteus . Proc. R. Soc. Lond. B 267, 2375–2384 (2000)
Schluter, D. Experimental evidence that competition promotes divergence in adaptive radiation. Science 266, 798–801 (1994)
Schluter, D. Adaptive radiation in sticklebacks: trade-offs in feeding performance and growth. Ecology 76, 82–90 (1995)
Rundle, H. D., Nagel, L. N., Boughman, J. W. & Schluter, D. Natural selection and parallel speciation in sympatric sticklebacks. Science 287, 306–308 (2000)
McPhail, J. D. Ecology and evolution of sympatric sticklebacks (Gasterosteus): evidence for a species pair in Paxton Lake, Texada Island, British Columbia. Can. J. Zool. 70, 361–369 (1992)
Matthews, B., Marchinko, K. B., Bolnick, D. I. & Mazumder, A. Specialization of trophic position and habitat use by sticklebacks in an adaptive radiation. Ecology 91, 1025–1034 (2010)
McGee, M. D. & Wainwright, P. C. Convergent evolution as a generator of phenotypic diversity in threespine stickleback. Evolution 67, 1204–1208 (2013)
McGee, M. D., Schluter, D. & Wainwright, P. C. Functional basis of ecological divergence in sympatric stickleback. BMC Evol. Biol. 13, 277 (2013)
Gow, J. L., Peichel, C. L. & Taylor, E. B. Contrasting hybridization rates between sympatric three-spined sticklebacks highlight the fragility of reproductive barriers between evolutionarily young species. Mol. Ecol. 15, 739–752 (2006)
Hatfield, T. & Schluter, D. Ecological speciation in sticklebacks: environment-dependent hybrid fitness. Evolution 53, 866–873 (1999)
Rundle, H. D. A test of ecologically dependent postmating isolation between sympatric sticklebacks. Evolution 56, 322–329 (2002)
Gow, J. L., Peichel, C. L. & Taylor, E. B. Ecological selection against hybrids in natural populations of sympatric threespine sticklebacks. J. Evol. Biol. 20, 2173–2180 (2007)
Marchinko, K. B. Predation’s role in repeated phenotypic and genetic divergence of armor in threespine stickleback. Evolution 63, 127–138 (2009)
Carlson, S. M., Kottas, A. & Mangel, M. Bayesian analysis of size-dependent overwinter mortality from size-frequency distributions. Ecology 91, 1016–1024 (2010)
Candolin, U. & Voigt, H.-R. Correlation between male size and territory quality: consequence of male competition or predation susceptibility? Oikos 95, 225–230 (2001)
MacColl, A. D. C. Parasites may contribute to ‘magic trait’ evolution in the adaptive radiation of three-spined sticklebacks, Gasterosteus aculeatus (Gasterosteiformes: Gasterosteidae). Biol. J. Linn. Soc. 96, 425–433 (2009)
Rogers, S. M. et al. Genetic signature of adaptive peak shift in threespine stickleback. Evolution 66, 2439–2450 (2012)
Jones, F. C. et al. The genomic basis of adaptive evolution in threespine sticklebacks. Nature 484, 55–61 (2012)
Jones, F. C. et al. A genome-wide SNP genotyping array reveals patterns of global and repeated species-pair divergence in sticklebacks. Curr. Biol. 22, 83–90 (2012)
Phillips, P. C. Epistasis—the essential role of gene interactions in the structure and evolution of genetic systems. Nature Rev. Genet. 9, 855–867 (2008)
Mackay, T. F. C. Epistasis and quantitative traits: using model organisms to study gene–gene interactions. Nature Rev. Genet. 15, 22–33 (2014)
Whitlock, M. C., Phillips, P. C., Moore, F. B.-G. & Tonsor, S. J. Multiple fitness peaks and epistasis. Annu. Rev. Ecol. Syst. 26, 601–629 (1995)
Via, S. Natural selection in action during speciation. Proc. Natl Acad. Sci. USA 106, 9939–9946 (2009)
Hohenlohe, P. A. et al. Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags. PLoS Genet. 6, e1000862 (2010)
Feder, J. L., Egan, S. P. & Nosil, P. The genomics of speciation-with-gene-flow. Trends Genet. 28, 342–350 (2012)
Strasburg, J. L. et al. What can patterns of differentiation across plant genomes tell us about adaptation and speciation? Phil. Trans. R. Soc. B 367, 364–373 (2012)
Seehausen, O. et al. Genomics and the origin of species. Nature Rev. Genet. 15, 176–192 (2014)
Rice, W. R. & Hostert, E. E. Laboratory experiments on speciation: what have we learned in 40 years? Evolution 47, 1637–1653 (1993)
Schluter, D. & Conte, G. L. Genetics and ecological speciation. Proc. Natl Acad. Sci. USA 106, 9955–9962 (2009)
Egan, S. P. & Funk, D. J. Ecologically dependent postmating isolation between sympatric host forms of Neochlamisus bebbianae leaf beetles. Proc. Natl Acad. Sci. USA 106, 19426–19431 (2009)
McBride, C. S. & Singer, M. C. Field studies reveal strong postmating isolation between ecologically divergent butterfly populations. PLoS Biol. 8, e1000529 (2010)
Rundle, H. D. & Nosil, P. Ecological speciation. Ecol. Lett. 8, 336–352 (2005)
Presgraves, D. C. The molecular evolutionary basis of species formation. Nature Rev. Genet. 11, 175–180 (2010)
Fry, B. Stable Isotope Ecology (Springer, 2006)
van Ooijen, J. W. & Voorrips, R. E. JoinMap® 3.0: Software for the Calculation of Genetic Linkage Maps (Plant Research International, 2001)
Broman, K. W. & Sen, S. A Guide to QTL Mapping with R/qtl (Springer Science+Business Media, 2009)
Schluter, D. Estimating the form of natural selection on a quantitative trait. Evolution 42, 849–861 (1988)
Wootton, R. J. A Functional Biology of Sticklebacks (Univ. of California Press, 1984)
Post, D. M. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718 (2002)
R. Development Core Team. R: A Language and Environment for Statistical Computing (http://www.R-project.org/) (R Foundation for Statistical Computing, 2011)
Vander Zanden, M. J. & Vadeboncoeur, Y. Fishes as integrators of benthic and pelagic food webs in lakes. Ecology 83, 2152–2161 (2002)
Bolnick, D. I. et al. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28 (2003)
McIntyre, P. B. & Flecker, A. S. Rapid turnover of tissue nitrogen of primary consumers in tropical freshwaters. Oecologia 148, 12–21 (2006)
Harmon, L. J. et al. Evolutionary diversification in stickleback affects ecosystem functioning. Nature 458, 1167–1170 (2009)
Behm, J. E., Ives, A. R. & Boughman, J. W. Breakdown in postmating isolation and the collapse of a species pair through hybridization. Am. Nat. 175, 11–26 (2010)
Bolnick, D. I. Sympatric speciation in threespine stickleback: why not? Int. J. Ecol. 2011, 942847 (2011)
Hartigan, J. A. & Hartigan, P. M. The dip test of unimodality. Ann. Stat. 13, 70–84 (1985)
Maechler, M. Package ‘diptest’: Hartigan’s Dip Test Statistic for Unimodality, corrected code, v. 0.75-4 (http://CRAN.R-project.org/package=diptest) (CRAN: Comprehensive R Archive Network, 2011)
Schluter, D. Adaptive radiation in sticklebacks: size, shape, and habitat use efficiency. Ecology 74, 699–709 (1993)
Albert, A. Y. K. et al. The genetics of adaptive shape shift in stickleback: pleiotropy and effect size. Evolution 62, 76–85 (2008)
Rohlf, F. J. TpsDig2 (http://life.bio.sunysb.edu/morph/soft-dataacq.html) (Department of Ecology and Evolution, State Univ. of New York, 2006)
Rohlf, F. J. & Slice, D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Zool. 39, 40–59 (1990)
Dryden, I. L. Package ‘shapes’: Statistical Shape Analysis, v. 1.1-3 (http://CRAN.R-project.org/package=shapes) (CRAN: Comprehensive R Archive Network, 2009)
Arnegard, M. E. et al. Sexual signal evolution outpaces ecological divergence during electric fish species radiation. Am. Nat. 176, 335–356 (2010)
Peichel, C. L. et al. The genetic architecture of divergence between threespine stickleback species. Nature 414, 901–905 (2001)
Warton, D. I., Duursma, R. A., Falster, D. S. & Taskinen, S. SMATR 3—an R package for estimation and inference about allometric lines. Methods Ecol. Evol. 3, 257–259 (2012)
Smith, R. J. Use and misuse of the reduced major axis for line-fitting. Am. J. Phys. Anthropol. 140, 476–486 (2009)
Miller, A. Subset Selection in Regression, 2nd edn, Vol. 95 (Chapman & Hall/CRC, 2002)
Lumley, T. Package ‘leaps’: Regression Subset Selection, v. 2.9 (http://CRAN.R-project.org/package=leaps) (CRAN: Comprehensive R Archive Network, 2009)
Mallows, C. L. Some comments on C P . Technometrics 15, 661–675 (1973)
Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 19, 716–723 (1974)
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd edn (Springer, 2002)
Sambrook, J. & Russell, D. W. Molecular Cloning: A Laboratory Manual, 3rd edn (Cold Spring Harbor Laboratory Press, 2001)
Hadfield, J. D., Richardson, D. S. & Burke, T. Towards unbiased parentage assignment: combining genetic, behavioural and spatial data in a Bayesian framework. Mol. Ecol. 15, 3715–3730 (2006)
Hadfield, J. Package ‘MasterBayes’: ML and MCMC Methods for Pedigree Reconstruction and Analysis, v. 2.50 (http://cran.r-project.org/web/packages/MasterBayes/) (CRAN: Comprehensive R Archive Network, 2012)
Manichaikul, A., Dupuis, J., Sen, S. & Broman, K. W. Poor performance of bootstrap confidence intervals for the location of a quantitative trait locus. Genetics 174, 481–489 (2006)
Kosambi, D. D. The estimation of map distance from recombination values. Ann. Eugen. 12, 172–175 (1944)
Theil, H. Economic Forecasts and Policy, 2nd edn (North-Holland, 1961)
Engle, R. F. & Brown, S. J. Model selection for forecasting. Appl. Math. Comput. 20, 313–327 (1986)
Hothorn, T. et al. Package ‘lmtest’: Testing Linear Regression Models, v. 0.9-30 (http://CRAN.R-project.org/package=lmtest) (CRAN: Comprehensive R Archive Network, 2012)
Erickson, D. L., Fenster, C. B., Stenøien, H. K. & Price, D. Quantitative trait locus analyses and the study of evolutionary process. Mol. Ecol. 13, 2505–2522 (2004)
Acknowledgements
We thank J. Perez for counting gill rakers; C. Sather for performing lab work for SNP genotyping; and K. Broman, G. Coop, I. Goodliffe, A. Greenwood, P. Wainwright, M. White and M. Wund for constructive comments. Stable isotopes were analysed at the University of California, Davis, Stable Isotope Facility. Funding was provided by grants from the US National Institutes of Health (F32 GM086125 to M.E.A., R01 GM089733 to C.L.P. and D.S., and P50 HG002568 to C.L.P. and D.M.K.), the Natural Sciences and Engineering Research Council of Canada (to D.S.) and the Canada Foundation for Innovation (to D.S.).
Author information
Authors and Affiliations
Contributions
M.E.A., C.L.P. and D.S. designed, planned and oversaw the project. M.E.A. made the crosses, set up the experimental pond and coordinated all field and laboratory research. M.E.A., K.B.M., S.K., N.B. and S.B. conducted fieldwork and stable-isotope analysis. M.D.M. measured functional morphological traits. B.M. and M.E.A. measured and analysed gut contents. S.K., D.S. and M.E.A. performed landmark-based morphometric analyses. M.E.A. analysed relationships between all traits and trophic variation. F.C.J., Y.F.C. and D.M.K. designed the SNP genotyping array. M.E.A., G.L.C., C.L.P and D.S. analysed SNP genotypes. D.S. determined the genealogy of the mapping population on the basis of SNP genotypes. M.E.A., C.L.P. and D.S. performed linkage and QTL analysis. M.E.A., C.L.P. and D.S. tested the genetic architecture of niche divergence. M.E.A., C.L.P., D.S., M.D.M., B.M. and G.L.C. interpreted the results. M.E.A. wrote the paper with input from C.L.P. and D.S., who are co-senior authors. All other authors provided editorial comments and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Experimental pond used in the study.
a, Photograph of pond no. 4 at the experimental pond facility of the University of British Columbia (Vancouver, British Columbia, Canada), taken in autumn 2008, during the collection of F2 juveniles. b, Diagram of the pond profile. See Supplementary Discussion for details on pond history before this study.
Extended Data Figure 2 Feeding patterns in relation to isotope signatures.
Plots show relationships between ingested prey counts from all available F2 hybrids (n = 99) and stable-isotope data. a, Loess-smoothed surface (span = 0.75, second-degree polynomials) of predicted chironomid counts plotted on original isotope axes (δ13C, δ15N). As with all other count data plotted here, counts were transformed as ln (chironomids + 1) and mapped according to the coloured scale. PC1 (black arrow) and PC2 (white) are based on the entire isotope distribution (Fig. 1a). Individuals are plotted as points according to the presence (crosses) or absence (filled circles) of calanoid copepods in their digestive tracts. b–g, Linear or logistic regression, accordingly, of ingested prey count or presence/absence data (transformed as above) on the different axes through isotope space. b, Chironomid count against δ13C, linear regression, slope estimate = 0.415, R2 = 0.199, F1,97 = 24.1, P = 3.70 × 10−6. c, Chironomid presence against niche score, logistic regression, slope coefficient = 0.504, z = 2.23, P = 0.0255. d, Collembola presence against diet deviation score, logistic regression, slope coefficient = 1.25, z = 4.26, P = 2.03 × 10−5. e, Calanoid copepod count against δ15N, linear regression, slope estimate = 0.492, R2 = 0.0608, F1,97 = 6.28, P = 0.0139. f, Calanoid copepod presence against niche score, logistic regression, slope coefficient = −0.463, z = −1.84, P = 0.0651. g, Calanoid copepod presence against diet deviation score, logistic regression, slope coefficient = −0.958, z = −2.67, P = 0.00766.
Extended Data Figure 3 Linkage map showing QTLs for all traits.
All G. aculeatus chromosomes are represented by LGs in the complete linkage map for this study (LGs and chromosomes use the same numbering29; LGs with no mapped QTLs are omitted here). Map distances are indicated with a scale at the left of each LG in centimorgans (cM). Coloured bars (at the right) are 1.5-LOD confidence intervals for QTL position (red bars, component traits of niche use; blue bars, other traits; Supplementary Table 3 provides LOD scores, map positions of LOD peaks, and effect sizes). The given SNP identifiers (IDs) are only for reference to Supplementary Table 4, which provides published SNP data30. For clarity, every other ID is omitted for SNP 066–098, even though these markers are present in the map. Markers closest to candidate QTLs for genetic model comparisons are highlighted: red text, nearest to candidate QTLs for niche score; green boxes, diet deviation score. Numbered traits are the x and y coordinates of morphometric landmarks (indicated on the fish photo): 1, posterior midpoint caudal peduncle; 2, anterior insertion anal fin at first soft ray; 3, posteroventral corner ectocoracoid; 4, posterodorsal corner ectocoracoid; 5, anteriormost corner ectocoracoid; 6, anteroventral corner opercle; 7, posterodorsal corner opercle; 8, dorsal edge opercle–hyomandibular boundary; 9, dorsalmost extent preopercle; 10, posteroventral corner preopercle; 11, anteriormost extent preopercle along ventral silhouette; 12, posteroventral extent maxilla; 13, anterodorsal extent maxilla; 14, suture between nasal and frontal bones along dorsal silhouette; 15, anterior margin orbit; 16, posterior margin orbit; 17, ventral margin orbit (landmarks 15–17 placed in line with vertical or horizontal midpoint of eye); 18, posterior extent supraoccipital along dorsal silhouette; 19, anterior insertion dorsal fin at first soft ray.
Extended Data Figure 4 Shape variation among F2 hybrid groups.
Each overlaid pair of wireframe diagrams compares the mean body shape of individuals in one of three groups of F2 hybrids (B, L or A; shown in dark blue) with the relative mean shape of a reference group consisting of all other F2 hybrids (group membership shown in Fig. 1a). Using data for 19 Procrustes-superimposed and unbent landmarks (Extended Data Fig. 3), the wireframe diagrams were produced and plotted in MorphoJ v.1.04a, on the basis of discriminant function analysis (Supplementary Discussion). The shape differences represented here are magnified eightfold for easier visual comparison. Group sample sizes: n = 91 (B), n = 92 (L), n = 93 (A), n = 335 (reference group). See Supplementary Discussion for a detailed description of patterns of variation in several specific features of shape that can be interpreted from these data.
Extended Data Figure 5 Variation of additional traits among F2 hybrid groups.
Means ( ± 1 s.e.m.) of F2 hybrids in groups B, L and A (Fig. 1a) are shown for the following traits (using raw data for long gill rakers and size-corrected data for the other traits): a, number of long gill rakers (ANOVA, F2,279 = 1.756, P = 0.175); b, residual anterior epaxial muscle height (F2,246 = 5.219, P = 0.00603); c, residual anterior epaxial muscle width (F2,246 = 4.223, P = 0.0157); d, residual neurocranium outlever length (F2,246 = 13.36, P = 3.10 × 10−6); e, residual buccal cavity length (F2,246 = 12.26, P = 8.42 × 10−6); f, residual gape (F2,246 = 7.974, P = 4.41 × 10−4). Numbers in parentheses are values of n. Traits are illustrated in Fig. 2e–g. The data conformed reasonably well to parametric statistical assumptions; ANOVA was therefore used to test trait variation among categories.
Extended Data Figure 6 Relationships between F2 hybrid functional morphology and niche score.
For key functional morphological traits known to differ between wild Paxton benthics and limnetics, trait data from all available F2 hybrids are plotted against niche score and fitted with linear regression (raw data for gill raker counts; size-corrected data for other traits): a, number of long gill rakers (R2 = 0.0146; F1,629 = 9.32; P = 0.00236); b, number of short gill rakers (R2 = 0.0253; F1,629 = 16.30; P = 6.06 × 10−5); c, residual anterior epaxial muscle height (R2 = 0.0125; F1,552 = 7.00; P = 0.00804); d, residual anterior epaxial muscle width (R2 = 0.0189; F1,552 = 10.61; P = 0.00119); e, residual upper jaw protrusion length (R2 = 0.0580; F1,552 = 34.00; P = 9.40 × 10−9); f, residual lower jaw-opening inlever length (R2 = 0.0660; F1,615 = 43.43; P = 9.45 × 10−11). Traits are illustrated in Fig. 2e–g. Directions of benthic–limnetic divergence in Paxton Lake (arrows at left of plots, here and in Fig. 2a–d) are based on previously published studies16,18,19, combined with validating counts of long and short gill rakers for this study (data not shown).
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-4, a Supplementary Discussion and Supplementary References. (PDF 1680 kb)
Rights and permissions
About this article
Cite this article
Arnegard, M., McGee, M., Matthews, B. et al. Genetics of ecological divergence during speciation. Nature 511, 307–311 (2014). https://doi.org/10.1038/nature13301
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13301
This article is cited by
-
Genetic architecture of a pollinator shift and its fate in secondary hybrid zones of two Petunia species
BMC Biology (2023)
-
The Dynamic Ontogenetic Shape Patterns of Adaptive Divergence and Sexual Dimorphism
Evolutionary Biology (2023)
-
Assessing the Levels of Functional Adaptation: Finite Element Analysis Reveals Species, Hybrid, and Sexual Variation in the Biomechanics of African Cichlid Mandibles
Evolutionary Biology (2022)
-
Multivariate analysis of morphology, behaviour, growth and developmental timing in hybrids brings new insights into the divergence of sympatric Arctic charr morphs
BMC Ecology and Evolution (2021)
-
Contrasting ecological niches lead to great postzygotic ecological isolation: a case of hybridization between carnivorous and herbivorous cyprinid fishes
Frontiers in Zoology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.