Abstract
Pluripotent stem cells provide a potential solution to current epidemic rates of heart failure1 by providing human cardiomyocytes to support heart regeneration2. Studies of human embryonic-stem-cell-derived cardiomyocytes (hESC-CMs) in small-animal models have shown favourable effects of this treatment3,4,5,6,7. However, it remains unknown whether clinical-scale hESC-CM transplantation is feasible, safe or can provide sufficient myocardial regeneration. Here we show that hESC-CMs can be produced at a clinical scale (more than one billion cells per batch) and cryopreserved with good viability. Using a non-human primate model of myocardial ischaemia followed by reperfusion, we show that cryopreservation and intra-myocardial delivery of one billion hESC-CMs generates extensive remuscularization of the infarcted heart. The hESC-CMs showed progressive but incomplete maturation over a 3-month period. Grafts were perfused by host vasculature, and electromechanical junctions between graft and host myocytes were present within 2 weeks of engraftment. Importantly, grafts showed regular calcium transients that were synchronized to the host electrocardiogram, indicating electromechanical coupling. In contrast to small-animal models7, non-fatal ventricular arrhythmias were observed in hESC-CM-engrafted primates. Thus, hESC-CMs can remuscularize substantial amounts of the infarcted monkey heart. Comparable remuscularization of a human heart should be possible, but potential arrhythmic complications need to be overcome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
11 June 2014
Savannah M. Cook has been added to the author list and an incorrect grant number has been updated.
References
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012)
Laflamme, M. A. & Murry, C. E. Heart regeneration. Nature 473, 326–335 (2011)
Caspi, O. et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J. Am. Coll. Cardiol. 50, 1884–1893 (2007)
van Laake, L. W. et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. (Amst.) 1, 9–24 (2007)
Laflamme, M. A. et al. Formation of human myocardium in the rat heart from human embryonic stem cells. Am. J. Pathol. 167, 663–671 (2005)
Fernandes, S. et al. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J. Mol. Cell. Cardiol. 49, 941–949 (2010)
Shiba, Y. et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322–325 (2012)
Laflamme, M. A. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotechnol. 25, 1015–1024 (2007)
Blin, G. et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J. Clin. Invest. 120, 1125–1139 (2010)
Bel, A. et al. Composite cell sheets: a further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells. Circulation 122, S118–S123 (2010)
Yang, X., Pabon, L. & Murry, C. E. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 114, 511–523 (2014)
Mauritz, C. et al. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur. Heart J. 32, 2634–2641 (2011)
Schwartz, S. D. et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379, 713–720 (2012)
Bretzner, F., Gilbert, F., Baylis, F. & Brownstone, R. M. Target populations for first-in-human embryonic stem cell research in spinal cord injury. Cell Stem Cell 8, 468–475 (2011)
Robey, T. E., Saiget, M. K., Reinecke, H. & Murry, C. E. Systems approaches to preventing transplanted cell death in cardiac repair. J. Mol. Cell. Cardiol. 45, 567–581 (2008)
Xu, C. et al. Efficient generation and cryopreservation of cardiomyocytes derived from human embryonic stem cells. Regen. Med. 6, 53–66 (2011)
Hoshino, T., Fujiwara, H., Kawai, C. & Hamashima, Y. Myocardial fiber diameter and regional distribution in the ventricular wall of normal adult hearts, hypertensive hearts and hearts with hypertrophic cardiomyopathy. Circulation 67, 1109–1116 (1983)
Gandolfi, F. et al. Large animal models for cardiac stem cell therapies. Theriogenology 75, 1416–1425 (2011)
van der Spoel, T. I. et al. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc. Res. 91, 649–658 (2011)
Kehat, I. et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nature Biotechnol. 22, 1282–1289 (2004)
Chen, H. S., Kim, C. & Mercola, M. Electrophysiological challenges of cell-based myocardial repair. Circulation 120, 2496–2508 (2009)
Dixon, J. A. & Spinale, F. G. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail 2, 262–271 (2009)
Weyers, J. J. et al. Effects of cell grafting on coronary remodeling after myocardial infarction. J. Am. Heart Assoc. 2, e000202 (2013)
Gantz, J. A. et al. Targeted genomic integration of a selectable floxed dual fluorescence reporter in human embryonic stem cells. PLoS ONE 7, e46971 (2012)
Xu, C. et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnol. 19, 971–974 (2001)
Zhu, W. Z., Van Biber, B. & Laflamme, M. A. Methods for the derivation and use of cardiomyocytes from human pluripotent stem cells. Methods Mol. Biol. 767, 419–431 (2011)
Hockemeyer, D. et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nature Biotechnol. 27, 851–857 (2009)
Biermann, M. et al. Differential effects of cytochalasin D and 2,3 butanedione monoxime on isometric twitch force and transmembrane action potential in isolated ventricular muscle: implications for optical measurements of cardiac repolarization. J. Cardiovasc. Electrophysiol. 9, 1348–1377 (1998)
Laurita, K. R. & Singal, A. Mapping action potentials and calcium transients simultaneously from the intact heart. Am. J. Physiol. Heart Circ. Physiol. 280, H2053–H2060 (2001)
Chong, J. J. H. et al. Progenitor cells identified by PDGFR-α expression in the developing and diseased human heart. Stem Cells Dev. 22, 1932–1943 (2013)
Acknowledgements
We thank S. Dupras, B. Brown, D. Rocha, E. Wilson, C. English, J. Randolph-Habecker and T. Goodpaster for assistance with these experiments. This work was supported by National Institutes of Health grants P01HL094374, R01HL084642, U01HL100405 and P01GM081619 and an Institute of Translational Health Sciences/Primate Center Ignition Award. J.J.H.C. was supported by National Health and Medical Research Council of Australia Overseas Training and Australian-American Fulbright Commission Fellowships. X.Y. is supported by an American Heart Association post-doctoral scholarship 12POST11940060. J.J.W. is supported by an American Heart Association post-doctoral scholarship 12POST9330030. H.-P.K. is a Markey Molecular Medicine investigator and the recipient of the Jose Carreras/E.D. Thomas Chair for Cancer Research.
Author information
Authors and Affiliations
Contributions
J.J.H.C., X.Y., C.W.D., E.M., L.P., H.R., H.-P.K., M.A.L. and C.E.M. designed the study. J.J.H.C. and E.M. performed mouse transplantation experiments. J.J.H.C. developed telemetry and analysed recordings. J.J.H.C., C.W.D., C.E.M., G.M.G., K.W.V., C.A.A., E.M. and V.N. performed macaque surgery and procedures. J.J.H.C., E.M., E.A.G. and C.E.H. performed echocardiography and E.M., E.G. and Y.-W.L. performed analysis. A.B. performed necropsies and non-cardiac histopathology. GCaMP3 visualization experiments were carried out and analysed by X.Y. and J.J.H.C. GCaMP3-expressing human ES cells were created by N.J.P., J.A.G. and B.V.B. hESC-CM production was by J.J.H.C., B.V.B., S.M.C., J.A.F. and M.A.L. Microcomputed tomography experiments were performed by J.J.W. and W.M.M. Jr. Immunohistochemistry was performed and analysed by J.J.H.C., V.M. and Y.-W.L. Figures were created by J.J.H.C. with assistance from X.Y., J.J.W., Y.-W.L., N.J.P. and V.M. The manuscript was written principally by J.J.H.C. and C.E.M.
Corresponding author
Ethics declarations
Competing interests
C.E.M. and M.A.L. are equity holders in BEAT BioTherapeutics.
Extended data figures and tables
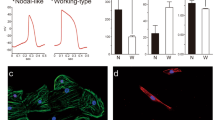
Extended Data Figure 1 Cryopreservation does not affect hESC-CM engraftment.
a, Schematic representation of experimental design for cryopreservation testing experiments. b, Human genomes detected after injection of cryopreserved or non-cryopreserved hESC-CMs were not significantly different (P > 0.05, t-test). Mean ± s.e.m. is shown (n = 9 biological replicates) Experiment was performed once. NS, not significant.
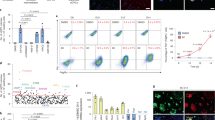
Extended Data Figure 2 Creation and validation of the GCaMP3-expressing human ES-cell lines.
a, Targeting construct for ZFN engineering of GCaMP3 into the AAVS1 locus. The endogenous genomic probe and neomycin resistance gene probe binding sites used for Southern blotting are shown. b, Southern blot analysis demonstrates a single integration event by hybridization for neomycin resistance cassette (left) and heterozygous AAVS1 integration by genomic probe labelling (right).
Extended Data Figure 3 Chromosomal analysis of human ES cells modified to encode GCaMP3.
a, H7-GCaMP3 ES-cell line demonstrates an isochrome of the chromosome 20 long arm (arrow). b, RUES2-GCaMP3 ES-cell line shows normal karyotype.
Extended Data Figure 4 Flow cytometry for cardiomyocyte differentiation of human ES cells.
Representative histogram of hESC-CMs after differentiation shows 73% cTnT-expressing cells.
Extended Data Figure 5 Schematic representation of experimental design.
Myocardial infarction was created by advancing a balloon catheter into the distal left anterior descending artery and inflating it to create ischaemia (90 min) followed by reperfusion. The infarct was induced 14 days (D) before hESC-CM delivery via left thoracotomy. Immunosuppression using cyclosporine A, methylprednisolone and abatacept (T-cell co-stimulatory antagonizing fusion protein) was delivered 5 days before cell delivery and continued until animals were euthanized. Primary endpoints were (1) histologically based morphometric calculations of infarct and graft size with analysis of graft composition, and (2) ex vivo analysis of graft–host electromechanical coupling enabled by GCaMP3 fluorescence detection. Secondary endpoints were (1) detection of arrhythmias by telemetric electrocardiogram analysis, and (2) analysis of left ventricular functional change by trans-oesophageal echocardiography.
Extended Data Figure 6 Technique for hESC-CM injection to infarct region and border zones using ‘mattress’ suture strategy.
a, The macaque infarcted ventricular apex is seen as a blanched region (dotted line) during left thoracotomy. A total of 15 aliquots, each containing 100 μl of hESC-CM in pro-survival cocktail, were delivered through five epicardial puncture sites (arrows, note one further puncture site not seen is on posterior aspect). b, hESC-CM retention after injection was increased by use of a ‘mattress’ suture. Crosses indicate insertion points of suture with dotted lines representing path of suture (exaggerated size for diagrammatic representation). A needle tip was inserted into the resulting rectangular area and the suture was tightened after a series of three injections (altering the trajectory of the needle) but before withdrawal of needle tip. c, Quantification of India ink retention after injection into left ventricular myocardium of anaesthetized macaques with or without use of the mattress suture technique (n = 3 biological replicates each group). A trend favouring greater retention with the mattress suture is seen.
Extended Data Figure 7 Remuscularization of the infarcted macaque heart.
a–f, Single channels of confocal immunofluorescence shown in Fig. 1a–f. Macaque heart shown was subjected to myocardial infarction and transplantation of hESC-CMs 14 days before being euthanized. g–l, Picrosirius Red staining of sections in close proximity to confocal immunofluorescence in a–f shows lack of fibrosis within hESC-CM grafts. Scale bars: 2,000 μm (a, f, g, l); 1,000 μm (b–e, h–k).
Extended Data Figure 8 Remuscularized infarct region is composed of engrafted cardiomyocytes that increase in size with time.
a, Quantification of the sarcomeric protein α-actinin expression in GFP-expressing grafts. The vast majority (>98%) of GFP-expressing cells co-expressed α-actinin. P3–P6 represent individual animals (n = 1) euthanized at 2 weeks (2 wk) 1 month (m) or 3 months after hESC-CM delivery. Five hundred to seven hundred cells were counted from three different graft regions of each heart. Percentage of GFP/α-actinin double-positive cells and GFP-positive/α-actinin-negative cells are shown as mean ± s.d. b, Normal curve from histograms showing the distribution of human ES-cell-derived cardiomyocyte diameters (graft) in monkey hearts 2 weeks, 1 or 3 months after cell delivery. c–f, Individual histograms with superimposed normal curve of animals P3–P6 (as above).
Extended Data Figure 9 No evidence of human graft rejection.
a–i, Representative low- (d–f) and high- (a–c and g–i) power magnification of hESC-CM graft 28 days after cell delivery to infarcted macaque heart. Representative low- (j–k) and high- (l, m) power magnification of infarct region from control macaque 28 days after sham treatment. The hESC-CM graft is detected by anti-GFP primary antibody with 3,3′-diaminobenzidine (DAB) detection of secondary antibody (brown). Few CD3+ T lymphocytes or CD20+ B lymphocytes are seen surrounding the hESC-CM grafts. Comparable numbers of T and B cells are seen in control infarcts receiving no hESC-CM treatment. Boxed inset regions show areas of higher magnification.
Extended Data Figure 10 Summary of ventricular tachycardia and echocardiographic assessment of left ventricular function.
a, Table characterizing episodes of ventricular tachycardia after engraftment of hESC-CMs (detailed in Fig. 5). Note that P5 demonstrated no discernible sinus rhythm on telemetric recording of the ECG 14 days after hESC-CM delivery. Although QRS morphology varied, the tachyarrhythmia comprised sustained periods of stable monomorphic QRS morphology. LBBB, left bundle branch block; RBBB, right bundle branch block. bpm, beats per minute. b, Left ventricular function was assessed by trans-oesophageal echocardiography at the following time points: before myocardial infarct creation, before hESC-CM delivery (2 weeks after myocardial infarction) and before animals were euthanized (2, 4 or 12 weeks after myocardial infarction). P7 received no cells/vehicle only. All other animals received hESC-CMs. Results shown are for left ventricular ejection fraction calculated by two blinded cardiologists from the two-chamber view of the left ventricle. Note that this view best captures the infarcted antero-apical wall. The vehicle-treated control monkey showed a modest diminution in ejection fraction post-infarction. The cell-treated animals showed variable responses, with some having increased function and some having decreased function. Because of small group size, no statistical effects of hESC-CM therapy can be discerned. c, Table of antibodies used. DSHB, Developmental Studies Hybridoma Bank.
Supplementary information
GCaMP3-expressing RUES2hESC-CMs exhibit robust fluorescence in vitro
Representative GCaMP3-expressing hESC-CMs were imaged using an inverted stereo-microscope with halogen and laser light sources. The video file shows the cardiomyocytes first by bright-field microscopy alone and then later with the green fluorescent signal. Note that the cells exhibit robust fluorescent transients with each contractile cycle. (MOV 4315 kb)
GCaMP3 expressing H7hESC-CMs exhibit robust fluorescence in vitro
Similar to Video S1, representative H7GCAMP3-hESC-CMs are shown first in bright field then with the green fluorescent signal. Note robust fluorescence with each cardiac cycle. (MOV 3327 kb)
Blood vessels extend from the host coronary network into the graft
3-dimensional rendering of microcomputed tomography (same heart shown in Extended Data Fig. 9) animated with rotation to enable better visualization of the vessels within the graft. Arteries feeding the graft are red, other vessels are gray in the uninjured cardiac tissue, or white within the graft. (MOV 22355 kb)
Modified Langendorff apparatus and GCAMP3 fluorescence imaging equipment
A harvested macaque heart is seen spontaneously beating after retrograde perfusion of with modified Tyrode’s solution. Electrodes are attached to the heart for electrocardiogram recording and analysis. Placement of the heart under a stereomicroscope with fluorescence imaging capabilities allows 2 visualization and analysis of epicardial fluorescence emitted from GCAMP3 expressing hESC-CM grafts. (MOV 23764 kb)
Intravital imaging of the infarcted macaque heart with GCaMP3-expressing hESC-CM grafts
A representative macaque heart with numerous GCaMP3-expressing hESC-CM graft regions, harvested at 28 days posttransplantation, mounted ex vivo on a Langendorff apparatus and mechanically arrested with 2,3-butanedione monoxime. This low power video (0.8x) shows several regions of GCaMP3-positive graft (distributed through infarct region and border zones) visible from the epicardial surface. All regions exhibited cyclic fluorescent transients that occurred in synchrony with QRS complexes of the host ECG, indicating 1:1 host-graft coupling. This video corresponds to Figure 2 b in main text. (MOV 6533 kb)
Higher power of intravital imaging of the infarcted macaque heart with GCaMP3-expressing hESC-CM graft
This video shows the same heart as video S3 at higher (2x) magnification and corresponds to Figure 2c-d in main text. (MOV 6527 kb)
Rights and permissions
About this article
Cite this article
Chong, J., Yang, X., Don, C. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277 (2014). https://doi.org/10.1038/nature13233
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13233
This article is cited by
-
Mature human induced pluripotent stem cell-derived cardiomyocytes promote angiogenesis through alpha-B crystallin
Stem Cell Research & Therapy (2023)
-
Recent advances in regulating the proliferation or maturation of human-induced pluripotent stem cell-derived cardiomyocytes
Stem Cell Research & Therapy (2023)
-
Optogenetic control of YAP can enhance the rate of wound healing
Cellular & Molecular Biology Letters (2023)
-
Harnessing stem cell and lineage reprogramming technology to treat cardiac fibrosis
Cell Regeneration (2023)
-
Regeneration of the heart: from molecular mechanisms to clinical therapeutics
Military Medical Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.