Abstract
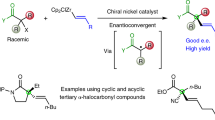
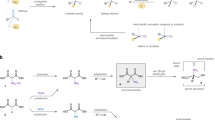
Small molecules that contain all-carbon quaternary stereocentres—carbon atoms bonded to four distinct carbon substituents—are found in many secondary metabolites and some pharmaceutical agents. The construction of such compounds in an enantioselective fashion remains a long-standing challenge to synthetic organic chemists. In particular, methods for synthesizing quaternary stereocentres that are remote from other functional groups are underdeveloped. Here we report a catalytic and enantioselective intermolecular Heck-type reaction of trisubstituted-alkenyl alcohols with aryl boronic acids. This method provides direct access to quaternary all-carbon-substituted β-, γ-, δ-, ε- or ζ-aryl carbonyl compounds, because the unsaturation of the alkene is relayed to the alcohol, resulting in the formation of a carbonyl group. The scope of the process also includes incorporation of pre-existing stereocentres along the alkyl chain, which links the alkene and the alcohol, in which the stereocentre is preserved. The method described allows access to diverse molecular building blocks containing an enantiomerically enriched quaternary centre.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
Data deposits
Data for the crystallized product (a derivative of 2f) have been deposited in the Cambridge Crystallographic Data Centre under accession number CCDC 988090.
References
Trost, B. M. & Jiang, C. Catalytic enantioselective construction of all-carbon quaternary stereocenters. Synthesis 369–396 (2006)
Douglas, C. J. & Overman, L. E. Catalytic asymmetric synthesis of all-carbon quaternary stereocenters. Proc. Natl Acad. Sci. USA 101, 5363–5367 (2004)
Christoffers, J. & Mann, A. Enantioselective construction of quaternary stereocenters. Angew. Chem. Int. Ed. 40, 4591–4597 (2001)
Das, J. P. & Marek, I. Enantioselective synthesis of all-carbon quaternary stereogenic centers in acyclic systems. Chem. Commun. 47, 4593–4623 (2011)
Marek, I. et al. All-carbon quaternary stereogenic centers in acyclic systems through the creation of several C–C bonds per chemical step. J. Am. Chem. Soc. 136, 2682–2694 (2014)
Masarwa, A. et al. Merging allylic carbon-hydrogen and selective carbon-carbon bond activation. Nature 505, 199–203 (2014)
Liu, W.-B., Reeves, C. M. & Stoltz, B. M. Enantio-, diastereo-, and regioselective iridium-catalyzed asymmetric allylic alkylation of acyclic β-ketoesters. J. Am. Chem. Soc. 135, 17298–17301 (2013)
Krautwald, S., Sarlah, D., Schafroth, M. A. & Carreira, E. M. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science 340, 1065–1068 (2013)
Taylor, M. S. & Jacobsen, E. N. Enantioselective Michael additions to α,β-unsaturated imides catalyzed by a salen-Al complex. J. Am. Chem. Soc. 125, 11204–11205 (2003)
Mermerian, A. H. & Fu, G. C. Catalytic enantioselective synthesis of quaternary stereocenters via intermolecular C-acylation of silyl ketene acetals: dual activation of the electrophile and the nucleophile. J. Am. Chem. Soc. 125, 4050–4051 (2003)
Sawamura, M., Hamashima, H. & Ito, Y. Catalytic asymmetric synthesis with trans-chelating chiral diphosphine ligand TRAP: rhodium-catalyzed asymmetric Michael addition of α-cyano carboxylates. J. Am. Chem. Soc. 114, 8295–8296 (1992)
Mauleón, P. & Carretero, J. C. Enantioselective construction of stereogenic quaternary centers via Rh-catalyzed asymmetric addition of alkenylboronic acids to α,β-unsaturated pyridylsulfones. Chem. Commun. 4961–4963 (2005)
Wu, J., Mampreian, D. M. & Hoveyda, A. H. Enantioselective synthesis of nitroalkanes bearing all-carbon quaternary stereogenic centers through Cu-catalyzed asymmetric conjugate additions. J. Am. Chem. Soc. 127, 4584–4585 (2005)
Hawner, C. et al. Rhodium-catalyzed asymmetric 1,4-addition of aryl alanes to trisubstituted enones: BINAP as an effective ligand in the formation of quaternary stereocenters. Angew. Chem. Int. Ed. 49, 7769–7772 (2010)
Shintani, R., Takeda, M., Nishimura, T. & Hayashi, T. Chiral tetrafluorobenzobarrelenes as effective ligands for rhodium-catalyzed asymmetric 1,4-addition of arylboroxines to β,β-disubstituted α,β-unsaturated ketones. Angew. Chem. Int. Ed. 49, 3969–3971 (2010)
Dabrowski, J. A., Villaume, M. T. & Hoveyda, A. H. Enantioselective synthesis of quaternary carbon stereogenic centers through copper-catalyzed conjugate additions of aryl- and alkylaluminum reagents to acyclic trisubstituted enones. Angew. Chem. Int. Ed. 52, 8156–8159 (2013)
Jung, B. & Hoveyda, A. H. Site- and enantioselective formation of allene-bearing tertiary or quaternary carbon stereogenic centers through NHC–Cu-catalyzed allylic substitution. J. Am. Chem. Soc. 134, 1490–1493 (2012)
Lee, Y. & Hoveyda, A. H. Lewis base activation of Grignard reagents with N-heterocyclic carbenes. Cu-free catalytic enantioselective additions to γ-chloro-α,β-unsaturated esters. J. Am. Chem. Soc. 128, 15604–15605 (2006)
Luchaco-Cullis, C. A., Mizutani, H., Murphy, K. E. & Hoveyda, A. H. Modular pyridinyl peptide ligands in asymmetric catalysis: enantioselective synthesis of quaternary carbon atoms through copper-catalyzed allylic substitutions. Angew. Chem. Int. Ed. 40, 1456–1460 (2001)
Denmark, S. E. & Fu, J. Catalytic, enantioselective addition of substituted allylic trichlorosilanes using a rationally-designed 2,2′-bispyrrolidine-based bisphosphoramide. J. Am. Chem. Soc. 123, 9488–9489 (2001)
Shi, W.-J. et al. Highly enantioselective hydrovinylation of α-alkyl vinylarenes. An approach to the construction of all-carbon quaternary stereocenters. J. Am. Chem. Soc. 128, 2780–2781 (2006)
Zhang, A. &. RajanBabu, T. V. All-carbon quaternary centers via catalytic asymmetric hydrovinylation. New approaches to the exocyclic side chain stereochemistry problem. J. Am. Chem. Soc. 128, 5620–5621 (2006)
Zhang, P., Le, H., Kyne, R. E. & Morken, J. P. Enantioselective construction of all-carbon quaternary centers by branch-selective Pd-catalyzed allyl-allyl cross-coupling. J. Am. Chem. Soc. 133, 9716–9719 (2011)
Mei, T.-S., Werner, E. W., Burckle, A. J. & Sigman, M. S. Enantioselective redox-relay oxidative Heck arylations of acyclic alkenyl alcohols using boronic acids. J. Am. Chem. Soc. 135, 6830–6833 (2013)
Werner, E. W., Mei, T.-S., Burckle, A. J. & Sigman, M. S. Enantioselective Heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science 338, 1455–1458 (2012)
Sigman, M. S. & Werner, E. W. Imparting catalyst control upon classical palladium-catalyzed alkenyl C–H bond functionalization reactions. Acc. Chem. Res. 45, 874–884 (2012)
Beletskaya, I. P. & Cheprakov, A. V. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 100, 3009–3066 (2000)
Calò, V., Nacci, A., Monopoli, A. & Ferola, V. Palladium-catalyzed Heck arylations of allyl alcohols in ionic liquids: remarkable base effect on the selectivity. J. Org. Chem. 72, 2596–2601 (2007)
Bouquillon, S., Ganchegui, B., Estrine, B., Hénin, F. & Muzart, J. Heck arylation of allylic alcohols in molten salts. J. Organomet. Chem. 634, 153–156 (2001)
Dounay, A. B. & Overman, L. E. The asymmetric intramolecular Heck reaction in natural product total synthesis. Chem. Rev. 103, 2945–2964 (2003)
Shibasaki, M., Vogl, E. M. & Ohshima, T. Asymmetric Heck reaction. Adv. Synth. Catal. 346, 1533–1552 (2004)
Mc Cartney, D. & Guiry, P. J. The asymmetric Heck and related reactions. Chem. Soc. Rev. 40, 5122–5150 (2011)
Melpolder, J. B. & Heck, R. F. Palladium-catalyzed arylation of allylic alcohols with aryl halides. J. Org. Chem. 41, 265–272 (1976)
Dang, Y., Qu, S., Wang, Z.-X. & Wang, X. A Computational mechanistic study of an unprecedented Heck-type relay reaction: insight into the origins of regio- and enantioselectivities. J. Am. Chem. Soc. 136, 986–998 (2014)
Xu, L. et al. Mechanism, reactivity, and selectivity in palladium-catalyzed redox-relay Heck arylations of alkenyl alcohols. J. Am. Chem. Soc. 136, 1960–1967 (2014)
Oliveira, C. C., Angnes, R. A. & Correia, C. R. D. Intermolecular enantioselective Heck–Matsuda arylations of acyclic olefins: application to the synthesis of β-aryl-γ-lactones and β-aryl aldehydes. J. Org. Chem. 78, 4373–4385 (2013)
Kikushima, K., Holder, J. C., Gatti, M. & Stoltz, B. M. Palladium-catalyzed asymmetric conjugate addition of arylboronic acids to five-, six-, and seven-membered β-substituted cyclic enones: enantioselective construction of all-carbon quaternary stereocenters. J. Am. Chem. Soc. 133, 6902–6905 (2011)
Yoo, K. S. et al. Asymmetric intermolecular boron Heck-type reactions via oxidative palladium(II) catalysis with chiral tridentate NHC-amidate-alkoxide ligands. J. Org. Chem. 75, 95–101 (2010)
Yoo, K. S. et al. Asymmetric intermolecular Heck-type reaction of acyclic alkenes via oxidative palladium(II) catalysis. Org. Lett. 9, 3933–3935 (2007)
Yonehara, K. et al. Palladium-catalyzed asymmetric intermolecular arylation of cyclic or acyclic alkenes using phosphinite-oxazoline ligands derived from d-glucosamine. J. Organomet. Chem. 603, 40–49 (2000)
Werner, E. W. & Sigman, M. S. A highly selective and general palladium catalyst for the oxidative Heck reaction of electronically nonbiased olefins. J. Am. Chem. Soc. 132, 13981–13983 (2010)
Yoo, K. S., Yoon, C. H. & Jung, K. W. Oxidative palladium(II) catalysis: a highly efficient and chemoselective cross-coupling method for carbon−carbon bond formation under base-free and nitrogenous-ligand conditions. J. Am. Chem. Soc. 128, 16384–16393 (2006)
Du, X. et al. Mizoroki−Heck type reaction of organoboron reagents with alkenes and alkynes. A Pd(II)-catalyzed pathway with Cu(OAc)2 as an oxidant. Org. Lett. 3, 3313–3316 (2001)
Gligorich, K. M. & Sigman, M. S. Recent advancements and challenges of palladiumII-catalyzed oxidation reactions with molecular oxygen as the sole oxidant. Chem. Commun. 3854–3867 (2009)
Steinhoff, B. A., King, A. E. & Stahl, S. S. Unexpected roles of molecular sieves in palladium-catalyzed aerobic alcohol oxidation. J. Org. Chem. 71, 1861–1868 (2006)
Kochi, T., Hamasaki, T., Aoyama, Y., Kawasaki, J. & Kakiuchi, F. Chain-walking strategy for organic synthesis: catalytic cycloisomerization of 1,N-dienes. J. Am. Chem. Soc. 134, 16544–16547 (2012)
Johnson, L. K., Killian, C. M. & Brookhart, M. New Pd(II)- and Ni(II)-based catalysts for polymerization of ethylene and α-olefins. J. Am. Chem. Soc. 117, 6414–6415 (1995)
Larock, R. C., Leung, W.-Y. & Stolz-Dunn, S. Synthesis of aryl-substituted aldehydes and ketones via palladium-catalyzed coupling of aryl halides and non-allylic unsaturated alcohols. Tetrahedr. Lett. 30, 6629–6632 (1989)
Acknowledgements
We thank the US National Institutes of Health (NIGMS GM063540) for financial support.
Author information
Authors and Affiliations
Contributions
T.-S.M. and H.H.P. performed the experiments and analysed the data. T.-S.M. and M.S.S. designed the experiments. T.-S.M. and M.S.S. prepared this manuscript with feedback from H.H.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data - see Table of Content for details. (PDF 23771 kb)
Rights and permissions
About this article
Cite this article
Mei, TS., Patel, H. & Sigman, M. Enantioselective construction of remote quaternary stereocentres. Nature 508, 340–344 (2014). https://doi.org/10.1038/nature13231
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13231
This article is cited by
-
Enantioselective C–C cross-coupling of unactivated alkenes
Nature Catalysis (2023)
-
Multi-site programmable functionalization of alkenes via controllable alkene isomerization
Nature Chemistry (2023)
-
Pd-catalyzed diastereoselective 1,1-diarylation of 1,1-disubstituted alkenes enabling the modular synthesis of 1,1,2,2-tetraarylethanes
Science China Chemistry (2023)
-
Stereoselective synthesis through remote functionalization
Nature Synthesis (2022)
-
Synthesis of tri- and tetrasubstituted stereocentres by nickel-catalysed enantioselective olefin cross-couplings
Nature Catalysis (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.