Abstract
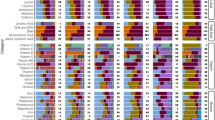
Dietary deficiencies of zinc and iron are a substantial global public health problem. An estimated two billion people suffer these deficiencies1, causing a loss of 63 million life-years annually2,3. Most of these people depend on C3 grains and legumes as their primary dietary source of zinc and iron. Here we report that C3 grains and legumes have lower concentrations of zinc and iron when grown under field conditions at the elevated atmospheric CO2 concentration predicted for the middle of this century. C3 crops other than legumes also have lower concentrations of protein, whereas C4 crops seem to be less affected. Differences between cultivars of a single crop suggest that breeding for decreased sensitivity to atmospheric CO2 concentration could partly address these new challenges to global health.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Tulchinsky, T. H. Micronutrient deficiency conditions: global health issues. Public Health Rev. 32, 243–255 (2010)
Caulfield, L. E. & Black, R. E. in Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attribution to Selected Major Risk Factors (eds Ezzati, M., Lopez, A. D., Rodgers, A. & Murray, C. J. L. ) Vol. 1, Ch. 5 (World Health Organization, 2004)
Stoltzfus, R. J., Mullany, L. & Black, R. E. in Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attribution to Selected Major Risk Factors (eds Ezzati, M., Lopez, A. D., Rodgers, A. & Murray, C. J. L. ) Vol. 1, Ch. 3 (World Health Organization, 2004)
De la Puente, L. S., Pérez, P. P., Martinez-Carrasco, R., Morcuende, R. M. & Del Molino, I. M. M. Action of elevated CO2 and high temperatures on the mineral chemical composition of two varieties of wheat. Agrochimica 44, 221–230 (2000)
Manderscheid, R., Bender, J., Jäger, H. J. & Weigel, H. J. Effects of season long CO2 enrichment on cereals. II. Nutrient concentrations and grain quality. Agric. Ecosyst. Environ. 54, 175–185 (1995)
Fangmeier, A., Grüters, U., Högy, P., Vermehren, B. & Jäger, H.-J. Effects of elevated CO2, nitrogen supply and tropospheric ozone on spring wheat. II. Nutrients (N, P, K, S, Ca, Mg, Fe, Mn, Zn). Environ. Pollut. 96, 43–59 (1997)
Pleijel, H. et al. Effects of elevated carbon dioxide, ozone and water availability on spring wheat growth and yield. Physiol. Plant. 108, 61–70 (2000)
Seneweera, S. P. & Conroy, J. P. Growth, grain yield and quality of rice (Oryza sativa L.) in response to elevated CO2 and phosphorus nutrition. Soil Sci. Plant Nutr. 43, 1131–1136 (1997)
Lieffering, M., Kim, H.-Y., Kobayashi, K. & Okada, M. The impact of elevated CO2 on the elemental concentrations of field-grown rice grains. Field Crops Res. 88, 279–286 (2004)
Prior, S. A., Runion, G. B., Rogers, H. H. & Torbert, H. A. Effects of atmospheric CO2 enrichment on crop nutrient dynamics under no-till conditions. J. Plant Nutr. 31, 758–773 (2008)
Högy, P. & Fangmeier, A. Atmospheric CO2 enrichment affects potatoes. 2. Tuber quality traits. Eur. J. Agron. 30, 85–94 (2009)
Högy, P. et al. Effects of elevated CO2 on grain yield and quality of wheat: results from a 3-year free-air CO2 enrichment experiment. Plant Biol. 11, 60–69 (2009)
Erbs, M. et al. Effects of free-air CO2 enrichment and nitrogen supply on grain quality parameters and elemental composition of wheat and barley grown in a crop rotation. Agric. Ecosyst. Environ. 136, 59–68 (2010)
Ainsworth, E. A. & Long, S. P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2 . New Phytol. 165, 351–372 (2005)
Curtis, P. S. & Wang, X. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113, 299–313 (1998)
Duval, B. D., Blankinship, J. C., Dijkstra, P. & Hungate, B. A. CO2 effects on plant nutrient concentration depend on plant functional group and available nitrogen: a meta-analysis. Plant Ecol. 213, 505–521 (2012)
Miller, L. V., Krebs, N. F. & Hambidge, M. K. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J. Nutr. 137, 135–141 (2007)
Fisher, B. S. et al. in Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Inter-governmental Panel on Climate Change (eds Metz, B. et al.) 169–250 (Cambridge Univ. Press, 2007)
Appel, L. J. et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. J. Am. Med. Assoc. 294, 2455–2464 (2005)
Millward, D. Joe. Identifying recommended dietary allowances for protein and amino acids: a critique of the 2007 WHO/FAO/UNU report. Br. J. Nutr. 108, S3–S21 (2012)
Swaminathan, S., Vaz, M. & Kurpad, A. V. Protein intakes in India. Br. J. Nutr. 108, S50–S58 (2012)
Leakey, A. Rising atmospheric carbon dioxide concentration and the future of C4 crops for food and fuel. Proc. R. Soc. Lond. B 276, 2333–2343 (2009)
Rogers, A., Ainsworth, E. A. & Leakey, A. D. Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiol. 151, 1009–1016 (2009)
Bloom, A. J. et al. CO2 enrichment inhibits shoot nitrate assimilation in C3 but not C4 plants and slows growth under nitrate in C3 plants. Ecology 93, 355–367 (2012)
Leakey, A. D. et al. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J. Exp. Bot. 60, 2859–2876 (2009)
Gifford, R., Barrett, D. & Lutze, J. The effects of elevated [CO2] on the C:N and C:P mass ratios of plant tissues. Plant Soil 224, 1–14, 10.1023/A:1004790612630. (2000)
McGrath, J. M. & Lobell, D. B. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant Cell Environ. 36, 697–705, 10.1111/pce.12007. (2013)
Monasterio, I. & Graham, R. D. Breeding for trace minerals in wheat. Food Nutr. Bull. 21, 392–396 (2000)
Searle, S. R., Casella, G. & McCulloch, C. E. Variance Components (Wiley, 1992)
Schenker, N. & Gentleman, J. F. On judging the significance of differences by examining the overlap between confidence intervals. Am. Stat. 55, 182–186 (2001)
Hasegawa, T. A. et al. Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Funct. Plant Biol. 40, 148–159 (2013)
Mollah, M., Norton, R. & Huzzey, J. Australian Grains Free Air Carbon dioxide Enrichment (AGFACE) facility: design and performance. Crop Pasture Sci. 60, 697–707 (2009)
Markelz, R., Strellner, R. & Leakey, A. Impairment of C4 photosynthesis by drought is exacerbated by limiting nitrogen and ameliorated by elevated CO2 in maize. J. Exp. Bot. 62, 3235–3246 (2011)
Gillespie, K. et al. Greater antioxidant and respiratory metabolism in field-grown soybean exposed to elevated O3 under both ambient and elevated CO2 . Plant Cell Environ. 35, 169–184 (2012)
Ottman, M. J. et al. Elevated CO2 increases sorghum biomass under drought conditions. New Phytol. 150, 261–273 (2001)
Sah, R. N. & Miller, R. O. Spontaneous reaction for acid dissolution of biological tissues in closed vessels. Anal. Chem. 64, 230–233 (1992)
AOAC. Official Method 972.43. in Official Methods of Analysis of AOAC International, 18th edition, Revision 1, 2006 Ch. 12 5–6 (AOAC International, 2006)
Mosse, J. Nitrogen to protein conversion factor for ten cereals and six legumes or oilseeds. A reappraisal of its definition and determination. Variation according to species and to seed protein content. J. Agric. Food Chem. 38, 18–24 (1990)
Haug, W. & Lantzsch, H. J. Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 34, 1423–1426 (1983)
Raboy, V. et al. Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1. Plant Physiol. 124, 355–368 (2000)
Wuehler, S. E., Peerson, J. M. & Brown, K. H. Use of national food balance data to estimate the adequacy of zinc in national food supplies: methodology and regional estimates. Public Health Nutr. 8, 812–819 (2005)
Acknowledgements
We thank L. S. De la Puente, M. Erbs, A. Fangmeier, P. Högy, M. Lieffering, R. Manderscheid, H. Pleijel and S. Prior for sharing data from their groups with us; H. Nakamura, T. Tokida, C. Zhu and S. Yoshinaga for contributions to the rice FACE project; and M. Hambidge, W. Willett, D. Schrag, K. Brown, R. Wessells, N. Fernando, J. Peerson and B. Kimball for reviews of earlier drafts or conceptual contributions to this project. V.R. thanks A. L. Harvey for her efforts in producing the phytate data included here. The National Agriculture and Food Research Organization (Japan) provided the grain samples of some rice cultivars. We thank the following for financial support of this work: the Bill & Melinda Gates Foundation; the Winslow Foundation; the Commonwealth Department of Agriculture (Australia), the International Plant Nutrition Institute, (Australia), the Grains Research and Development Corporation (Australia), the Ministry of Agriculture, Forestry and Fisheries (Japan); the National Science Foundation (NSF IOS-08-18435); USDA NIFA 2008-35100-044459; research at SoyFACE was supported by the US Department of Agriculture Agricultural Research Service; Illinois Council for Food and Agricultural Research (CFAR); Department of Energy’s Office of Science (BER) Midwestern Regional Center of the National Institute for Climatic Change Research at Michigan Technological University, under Award Number DEFC02- 06ER64158; and the National Research Initiative of Agriculture and Food Research Initiative Competitive Grants Program Grant no. 2010–65114–20343 from the USDA National Institute of Food and Agriculture. Early stages of this work received support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05).
Author information
Authors and Affiliations
Contributions
S.S.M. conceived the overall project and drafted the manuscript. A.Z., I.K., J.S. and P.H. performed statistical analyses. P.H. and A.D.B.L. provided substantial input into methods descriptions. A.J.B., E.C. and V.R. analysed grain samples for nutrient content. G.F., T.H., A.D.B.L., R.L.N., M.J.O., H.S., S.S., M.T. and Y.U. conducted FACE experiments and supplied grain for analysis. N.M.H. and P.H. assisted with elements of experimental design. K.A.S. and L.H.D. assisted with data collection and analysis. All authors contributed to manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
PowerPoint slides
Rights and permissions
About this article
Cite this article
Myers, S., Zanobetti, A., Kloog, I. et al. Increasing CO2 threatens human nutrition. Nature 510, 139–142 (2014). https://doi.org/10.1038/nature13179
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13179
This article is cited by
-
Combined effect of elevated CO2 and Fe deficiency on common bean metabolism and mineral profile
Plant and Soil (2024)
-
Elevated CO2 affects interspecific competition between the invasive thrips Frankliniella occidentalis and native thrips species
Journal of Pest Science (2024)
-
Transition metals for stabilizing lithium metal anode: advances and perspectives
Tungsten (2024)
-
Risks associated with global warming of 1.5 to 4 °C above pre-industrial levels in human and natural systems in six countries
Climatic Change (2024)
-
Enhancing sustainable development through plant genetics
Nature Reviews Genetics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.