Abstract
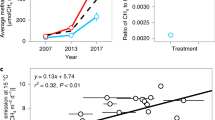
Methane (CH4) is an important greenhouse gas because it has 25 times the global warming potential of carbon dioxide (CO2) by mass over a century1. Recent calculations suggest that atmospheric CH4 emissions have been responsible for approximately 20% of Earth’s warming since pre-industrial times2. Understanding how CH4 emissions from ecosystems will respond to expected increases in global temperature is therefore fundamental to predicting whether the carbon cycle will mitigate or accelerate climate change. Methanogenesis is the terminal step in the remineralization of organic matter and is carried out by strictly anaerobic Archaea3. Like most other forms of metabolism, methanogenesis is temperature-dependent4,5. However, it is not yet known how this physiological response combines with other biotic processes (for example, methanotrophy6, substrate supply3,7, microbial community composition8) and abiotic processes (for example, water-table depth9,10) to determine the temperature dependence of ecosystem-level CH4 emissions. It is also not known whether CH4 emissions at the ecosystem level have a fundamentally different temperature dependence than other key fluxes in the carbon cycle, such as photosynthesis and respiration. Here we use meta-analyses to show that seasonal variations in CH4 emissions from a wide range of ecosystems exhibit an average temperature dependence similar to that of CH4 production derived from pure cultures of methanogens and anaerobic microbial communities. This average temperature dependence (0.96 electron volts (eV)), which corresponds to a 57-fold increase between 0 and 30°C, is considerably higher than previously observed for respiration (approximately 0.65 eV)11 and photosynthesis (approximately 0.3 eV)12. As a result, we show that both the emission of CH4 and the ratio of CH4 to CO2 emissions increase markedly with seasonal increases in temperature. Our findings suggest that global warming may have a large impact on the relative contributions of CO2 and CH4 to total greenhouse gas emissions from aquatic ecosystems, terrestrial wetlands and rice paddies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Forster, P. et al. in Climate Change 2007: the Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon, S. et al.) (Cambridge Univ. Press, 2007)
Kirschke, S. et al. Three decades of global methane sources and sinks. Nature Geosci. 6, 813–823 (2013)
Thauer, R. K., Kaster, A.-K., Seedorf, H., Buckel, W. & Hedderich, R. Methanogenic archaea: ecologically relevant differences in energy conservation. Nature Rev. Microbiol. 6, 579–591 (2008)
Westermann, P., Ahring, B. K. & Mah, R. A. Temperature compensation in Methanosarcina barkeri by modulation of hydrogen and acetate affinity. Appl. Environ. Microbiol. 55, 1262–1266 (1989)
Zinder, S. H., Anguish, T. & Cardwell, S. C. Effects of temperature on methanogenesis in a thermophilic (58°C) anaerobic digester. Appl. Environ. Microbiol. 47, 808–813 (1984)
Segers, R. Methane production and methane consumption: a review of processes underlying wetland methane fluxes. Biogeochemistry 41, 23–51 (1998)
Whiting, G. J. & Chanton, J. P. Primary production control of methane emission from wetlands. Nature 364, 794–795 (1993)
Fey, A. & Conrad, R. Effect of temperature on carbon and electron flow and on the archaeal community in methanogenic rice field soil. Appl. Environ. Microbiol. 66, 4790–4797 (2000)
Moore, T. R., Roulet, N. T. & Waddington, J. M. Uncertainty in predicting the effect of climatic change on the carbon cycling of Canadian peatlands. Clim. Change 40, 229–245 (1998)
Moore, T. R. & Roulet, N. T. Methane flux: water table relations in northern wetlands. Geophys. Res. Lett. 20, 587–590 (1993)
Yvon-Durocher, G. et al. Reconciling the temperature dependence of respiration across time scales and ecosystem types. Nature 487, 472–476 (2012)
Allen, A. P., Gillooly, J. F. & Brown, J. H. Linking the global carbon cycle to individual metabolism. Funct. Ecol. 19, 202–213 (2005)
Bridgham, S. D., Cadillo-Quiroz, H., Keller, J. K. & Zhuang, Q. Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Change Biol. 19, 1325–1346 (2013)
Bastviken, D., Tranvik, L. J., Downing, J. A., Crill, P. M. & Enrich-Prast, A. Freshwater methane emissions offset the continental carbon sink. Science 331, 50 (2011)
Gedney, N., Cox, P. M. & Huntingford, C. Climate feedback from wetland methane emissions. Geophys. Res. Lett. 31, L20503 (2004)
Riley, W. J. et al. Barriers to predicting changes in global terrestrial methane fluxes: analyses using CLM4Me, a methane biogeochemistry model integrated in CESM. Biogeosciences 8, 1925–1953 (2011)
Spahni, R. et al. Constraining global methane emissions and uptake by ecosystems. Biogeosciences 8, 1643–1665 (2011)
Wania, R., Ross, I. & Prentice, I. C. Implementation and evaluation of a new methane model within a dynamic global vegetation model: LPJ-WHyMe v1.3.1. Geosci. Mod. Dev. 3, 565–584 (2010)
Christensen, T. R. et al. Factors controlling large scale variations in methane emissions from wetlands. Geophys. Res. Lett. 30, 1414 (2003)
Crill, P. M. et al. Methane flux from Minnesota peatlands. Glob. Biogeochem. Cycles 2, 371–384 (1988)
Walter, B. P. & Heimann, M. A process-based, climate-sensitive model to derive methane emissions from natural wetlands: application to five wetland sites, sensitivity to model parameters, and climate. Glob. Biogeochem. Cycles 14, 745–765 (2000)
Eyring, H. The activated complex and the absolute rate of chemical reactions. Chem. Rev. 17, 65–77 (1935)
Yvon-Durocher, G., Montoya, J. M., Woodward, G., Jones, J. I. & Trimmer, M. Warming increases the proportion of primary production emitted as methane from freshwater mesocosms. Glob. Change Biol. 17, 1225–1234 (2011)
Bradford, M. A. et al. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol. Lett. 11, 1316–1327 (2008)
Melton, J. R. et al. Present state of global wetland extent and wetland methane modelling: conclusions from a model inter-comparison project (WETCHIMP). Biogeosciences 10, 753–788 (2013)
Zuur, A., Ieno, E., Walker, N. & Saveliev, A. Mixed Effects Models and Extensions in Ecology with R (Springer Verlag, 2009)
Bolker, B. M. et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 (2009)
R core development team R: a language and environment for statistical computing. http://www.R-project.org (R Foundation for Statistical Computing, 2014)
Pinheiro, J. & Bates, D. M. Mixed-effects models in S and S-PLUS (Springer Verlag, 2000)
Van den Berg, L., Patel, G. B., Clark, D. S. & Lentz, C. P. Factors affecting the rate of methane formation from acetic-acid by enriched methanogeneic cultures. Can. J. Microbiol. 22, 1312–1319 (1976)
Yao, H. & Conrad, R. Effect of temperature on reduction of iron and production of carbon dioxide and methane in anoxic wetland rice soils. Biol. Fertil. Soils 32, 135–141 (2000)
van Bodegom, P. M. & Stams, A. Effects of alternative electron acceptors and temperature on methanogenesis in rice paddy soils. Chemosphere 39, 167–182 (1999)
Lofton, D., Whalen, S. & Hershey, A. Effect of temperature on methane dynamics and evaluation of methane oxidation kinetics in shallow Arctic Alaskan lakes. Hydrobiologia 721, 209–222 (2014)
Duc, N. T., Crill, P. & Bastviken, D. Implications of temperature and sediment characteristics on methane formation and oxidation in lake sediments. Biogeochemistry 100, 185–196 (2010)
Megonigal, J. P. & Schlesinger, W. H. Methane-limited methanotrophy in tidal freshwater swamps. Glob. Biogeochem. Cycles 16, 1088–1095 (2002)
Lapierre, J.-F. & del Giorgio, P. A. Geographical and environmental drivers of regional differences in the lake pCO2 versus DOC relationship across northern landscapes. J. Geophys. Res.-Biogeosci. 117,, (2012)
Vachon, D., Prairie, Y. T. & Cole, J. J. The relationship between near-surface turbulence and gas transfer velocity in freshwater systems and its implications for floating chamber measurements of gas exchange. Limnol. Oceanogr. 55, 1723–1732 (2010)
Bastviken, D. et al. Methane emissions from Pantanal, South America, during the low water season: toward more comprehensive sampling. Environ. Sci. Technol. 44, 5450–5455 (2010)
Acknowledgements
We thank M. Trimmer for early discussions that inspired much of this work, as well as P. Cox and T. Lenton for comments on earlier drafts of the manuscript.
Author information
Authors and Affiliations
Contributions
G.Y.-D., D.B. and C.G. had initial discussions. G.Y.-D. conceived the study, analysed the data and wrote the first draft of the manuscript. D.B., P.A.d.G., C.G., N.T.-D., R.C. and A.S. contributed original data. A.P.A. wrote the theory for the CH4:CO2 temperature dependence. All authors contributed to revisions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
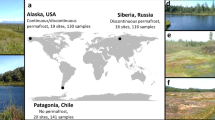
Extended Data Figure 1 Global distribution of the field sites included in our analysis of ecosystem-level CH4 emissions.
The size of each point relates to the logarithm (base 10) of the mean rate of CH4 emission (mg CH4 per m2 per day) over the duration of the experiment.
Extended Data Figure 2 Correlations of average site temperatures with average CH4 emissions and CH4 emissions at fixed temperature for globally distributed ecosystems.
Average CH4 emissions are shown in a–c, and CH4 emissions at fixed temperature are shown in d–f. Average site temperature is positively correlated with average CH4 emissions,  , for aquatic ecosystems (a) and natural wetlands (b), although temperature explains only 12% and 9% of the variance, respectively, for CH4 emissions from these two ecosystem types. In contrast, it is not significantly correlated with average CH4 emissions for rice paddies (c). Average site temperature is also not correlated with CH4 emissions at fixed temperature,
, for aquatic ecosystems (a) and natural wetlands (b), although temperature explains only 12% and 9% of the variance, respectively, for CH4 emissions from these two ecosystem types. In contrast, it is not significantly correlated with average CH4 emissions for rice paddies (c). Average site temperature is also not correlated with CH4 emissions at fixed temperature,  (where
(where  is the average temperature across the field emissions data set (15.6 °C)), for aquatic ecosystems (d) and rice paddies (f), but is significantly negatively correlated for natural wetlands (e). The latter finding suggests that temperature-dependent biotic (for example, methanotrophy, substrate supply, microbial community structure, physiological acclimation and/or adaptation) and abiotic factors (for example, water-table depth) may play an important role in constraining variation in total CH4 emissions among wetlands along geographic temperature gradients.
is the average temperature across the field emissions data set (15.6 °C)), for aquatic ecosystems (d) and rice paddies (f), but is significantly negatively correlated for natural wetlands (e). The latter finding suggests that temperature-dependent biotic (for example, methanotrophy, substrate supply, microbial community structure, physiological acclimation and/or adaptation) and abiotic factors (for example, water-table depth) may play an important role in constraining variation in total CH4 emissions among wetlands along geographic temperature gradients.
Supplementary information
Supplementary Information
This file contains Supplementary Information sections 1-2 and additional references. (PDF 697 kb)
Rights and permissions
About this article
Cite this article
Yvon-Durocher, G., Allen, A., Bastviken, D. et al. Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 507, 488–491 (2014). https://doi.org/10.1038/nature13164
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13164
This article is cited by
-
Relative increases in CH4 and CO2 emissions from wetlands under global warming dependent on soil carbon substrates
Nature Geoscience (2024)
-
Boreal–Arctic wetland methane emissions modulated by warming and vegetation activity
Nature Climate Change (2024)
-
Salinity causes widespread restriction of methane emissions from small inland waters
Nature Communications (2024)
-
Hourly methane and carbon dioxide fluxes from temperate ponds
Biogeochemistry (2024)
-
Dissolved greenhouse gas (CO2, CH4, and N2O) emissions from highland lakes of the Andes cordillera in Northern Ecuador
Aquatic Sciences (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.