Abstract
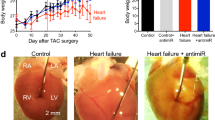
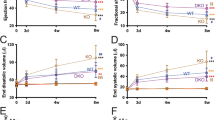
Heart failure is characterized by a debilitating decline in cardiac function1, and recent clinical trial results indicate that improving the contractility of heart muscle cells by boosting intracellular calcium handling might be an effective therapy2,3. MicroRNAs (miRNAs) are dysregulated in heart failure4,5 but whether they control contractility or constitute therapeutic targets remains speculative. Using high-throughput functional screening of the human microRNAome, here we identify miRNAs that suppress intracellular calcium handling in heart muscle by interacting with messenger RNA encoding the sarcoplasmic reticulum calcium uptake pump SERCA2a (also known as ATP2A2). Of 875 miRNAs tested, miR-25 potently delayed calcium uptake kinetics in cardiomyocytes in vitro and was upregulated in heart failure, both in mice and humans. Whereas adeno-associated virus 9 (AAV9)-mediated overexpression of miR-25 in vivo resulted in a significant loss of contractile function, injection of an antisense oligonucleotide (antagomiR) against miR-25 markedly halted established heart failure in a mouse model, improving cardiac function and survival relative to a control antagomiR oligonucleotide. These data reveal that increased expression of endogenous miR-25 contributes to declining cardiac function during heart failure and suggest that it might be targeted therapeutically to restore function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bonow, R. O., Mann, D. L., Zipes, D. P. & Libby, P. Braunwald’s Heart Disease (Saunders, 2011)
Meyer, M. et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation 92, 778–784 (1995)
Jessup, M. et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 124, 304–313 (2011)
Ikeda, S. et al. Altered microRNA expression in human heart disease. Physiol. Genomics 31, 367–373 (2007)
Leptidis, S. et al. A deep sequencing approach to uncover the miRNOME in the human heart. PLoS ONE 8, e57800 (2013)
Shah, A. M. & Mann, D. L. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet 378, 704–712 (2011)
Mudd, J. O. & Kass, D. A. Tackling heart failure in the twenty-first century. Nature 451, 919–928 (2008)
Greenstein, J. L. & Winslow, R. L. Integrative systems models of cardiac excitation–contraction coupling. Circ. Res. 108, 70–84 (2011)
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009)
Filipowicz, W., Bhattacharyya, S. N. & Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nature Rev. Genet. 9, 102–114 (2008)
Gurha, P. et al. Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction. Circulation 125, 2751–2761 (2012)
Kawase, Y. et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J. Am. Coll. Cardiol. 51, 1112–1119 (2008)
Shirdel, E. A., Xie, W., Mak, T. W. & Jurisica, I. NAViGaTing the micronome—using multiple microRNA prediction databases to identify signalling pathway-associated microRNAs. PLoS ONE 6, e17429 (2011)
Lemons, D., Maurya, M. R., Subramaniam, S. & Mercola, M. Developing microRNA screening as a functional genomics tool for disease research. Front. Physiol. 4, 223 (2013)
Cerignoli, F. et al. High throughput measurement of Ca2+ dynamics for drug risk assessment in human stem cell-derived cardiomyocytes by kinetic image cytometry. J. Pharmacol. Toxicol. Methods 66, 246–256 (2012)
Beuckelmann, D. J., Nabauer, M. & Erdmann, E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation 85, 1046–1055 (1992)
Piacentino, V., III et al. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ. Res. 92, 651–658 (2003)
Krützfeldt, J. et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438, 685–689 (2005)
Bonauer, A. et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 324, 1710–1713 (2009)
Helwak, A., Kudla, G., Dudnakova, T. & Tollervey, D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 153, 654–665 (2013)
Kho, C. et al. SUMO1-dependent modulation of SERCA2a in heart failure. Nature 477, 601–605 (2011)
Kho, C., Lee, A. & Hajjar, R. J. Altered sarcoplasmic reticulum calcium cycling—targets for heart failure therapy. Nature Rev. Cardiol. 9, 717–733 (2012)
Higazi, D. R. et al. Endothelin-1-stimulated InsP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol. Cell 33, 472–482 (2009)
Hulot, J. S. et al. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation 124, 796–805 (2011)
Fu, Y. et al. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am. J. Nephrol. 32, 581–589 (2010)
Schmidt, H. H., Wingler, K., Kleinschnitz, C. & Dusting, G. NOX4 is a Janus-faced reactive oxygen species generating NADPH oxidase. Circ. Res. 111, e15–16 (2012)
Dirkx, E. et al. Nfat and miR-25 cooperate to reactivate the transcription factor Hand2 in heart failure. Nature Cell Biol. 15, 1282–1293 (2013)
Colas, A. R. et al. Whole-genome microRNA screening identifies let-7 and mir-18 as regulators of germ layer formation during early embryogenesis. Genes Dev. 26, 2567–2579 (2012)
McKeithan, W. L., Colas, A. R., Bushway, P. J., Ray, S. & Mercola, M. Serum-free generation of multipotent mesoderm (Kdr+) progenitor cells in mouse embryonic stem cells for functional genomics screening. Curr. Protoc. Stem Cell Biol. Chapter 1,. 13 (2012)
Roy, S. et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc. Res. 82, 21–29 (2009)
Matkovich, S. J. et al. Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation 119, 1263–1271 (2009)
van Rooij, E. et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl Acad. Sci. USA 105, 13027–13032 (2008)
Cheng, Y. et al. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am. J. Pathol. 170, 1831–1840 (2007)
Tatsuguchi, M. et al. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J. Mol. Cell. Cardiol. 42, 1137–1141 (2007)
Sayed, D., Hong, C., Chen, I. Y., Lypowy, J. & Abdellatif, M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ. Res. 100, 416–424 (2007)
van Rooij, E. et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl Acad. Sci. USA 103, 18255–18260 (2006)
Zolotukhin, S. et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6, 973–985 (1999)
Andersson, K. B. et al. Moderate heart dysfunction in mice with inducible cardiomyocyte-specific excision of the Serca2 gene. J. Mol. Cell. Cardiol. 47, 180–187 (2009)
Swift, F. et al. Extreme sarcoplasmic reticulum volume loss and compensatory T-tubule remodeling after Serca2 knockout. Proc. Natl Acad. Sci. USA 109, 3997–4001 (2012)
Pacher, P., Nagayama, T., Mukhopadhyay, P., Batkai, S. & Kass, D. A. Measurement of cardiac function using pressure–volume conductance catheter technique in mice and rats. Nature Protocols 3, 1422–1434 (2008)
Bers, D. M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 70, 23–49 (2008)
Kockskämper, J. et al. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J. Mol. Cell. Cardiol. 45, 128–147 (2008)
Bootman, M. D. & Roderick, H. L. Why, where, and when do cardiac myocytes express inositol 1,4,5-trisphosphate receptors? Am. J. Physiol. Heart Circ. Physiol. 294, H579–H581 (2008)
Zima, A. V., Bare, D. J., Mignery, G. A. & Blatter, L. A. IP3-dependent nuclear Ca2+ signalling in the mammalian heart. J. Physiol. (Lond.) 584, 601–611 (2007)
Acknowledgements
We thank P. Aza-Blanc and F. Cerignoli; O. Kim, L. Liang and E. Kohlbrenner for their technical support; G. Christensen for providing the SERCA2a knockout mice; and H. el Azzouzi for TAC operations and histological sections. This work was supported by California Institute for Regenerative Medicine (RC1-000132), the National Institutes of Health (NIH) (R01HL113601, P01HL098053 and R01HL108176) and the Fondation Leducq to M.M.; by the NIH (NIH R01HL093183, R01HL088434, P20HL100396 and a Program of Excellence in Nanotechnology Contract no. HHSN26820100045C and P50HL112324) to R.J.H.; P30CA030199 and P30AR061303 for Sanford-Burnham Medical Research Institute functional genomics and cytometry. W.J.P. was supported by the Global Research Laboratory Program of the South Korean Government (M6-0605-00-0001). J.P.G.S. and P.A.F.D. were supported by the Netherlands Heart foundation and Project P1.05 LUST of the BioMedical Materials institute co-funded by the Dutch Ministry of Economic Affairs, Agriculture and Innovation. C.W. was supported by a fellowship from the Spanish National Research Council. A.v.M. was a Netherlands Heart Institute ICIN fellow.
Author information
Authors and Affiliations
Contributions
C.W., A.R.-M., D.J., R.J.H. and M.M. conceived and designed the project following an initial concept from M.M.; C.W., D.J., A.R.-M., C.K., A.L., S.M. and A.v.M. performed experiments and analysed the data; and interpreted results with M.M. and R.J.H. W.J.P. developed reagents for post-translational modification assays on SERCA2a. M.M., R.J.H., C.W., A.R.-M., D.J., A.v.M., P.A.F.D. and J.P.G.S. wrote and edited the manuscript. Major funding was obtained by P.A.F.D., R.J.H. and M.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Effect of miR-25 on IP3R1 and cardiomyocyte calcium transients in vitro.
a, Effect of miR-25, anti-miR-25 and siIP3R1 transfection on IP3R1 protein levels in HL-1 cells. b, Sequence of the putative miR-25 target recognition element in IP3R1 mRNA and the corresponding alteration by site-directed mutagenesis. Mutation abolished inhibition of the luciferase signal by miR-25 (n = 10). a, b, Data are represented as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001. NS, not significant (Student’s t-test). c, Representative Ca2+ transient of HL-1 cells transfected as indicated. Note that miR-25 slowed the repolarization phase kinetics, whereas anti-miR-25 quickened the kinetics. Co-transfection normalized kinetics. d, Kinetic imaging cytometry analysis of Ca2+ transient kinetics during the decay phase (Ca2+ transient duration time from 75% to 25% maximal value, CaTD75–25) of transfected NRVCs (n > 550 cells). Box defines interquartile range; bar shows median; whiskers show ± 5th and 95th percentile; dots indicate outliers. *P < 0.05, **P < 0.01, ***P < 0.001. NS, not significant (one-tailed ANOVA). Experiments were replicated two times.
Extended Data Figure 2 Effect of miR-25 on calcium handling proteins.
Expression of candidate targets of miR-25 in transfected HL-1 cells (lysates collected 5 days post-transfection). No effect of miR-25 or anti-miR-25 was noted for these proteins. Data are represented as mean ± s.e.m. Experiments were replicated two times.
Extended Data Figure 3 Calcium transient effects of miR-25 compared with that of siRNAs against SERCA2a and IP3R isoforms.
Cardiomyocyte-like HL-1 cells were transfected with miR-25, siRNA to SERCA2a (siSerca2a) (left panels) or siRNAs to IP3R1 or IP3R2 (siIP3R1, siIP3R2) (right panels), and analysed 72 h later by kinetic imaging cytometry. Kinetic parameters are CaTD50, CaTD75–25 and Vmax upstroke. Data are represented as whisker plots, with the box denoting the 25th and 75th percentiles, the whiskers the 5th and 95th percentiles, the middle bar the median, and outliers indicated as individual dots. Note that siSerca2a and miR-25 elicited comparable effects, both markedly delaying the Ca2+ uptake phase parameters CaTD50 and CaTD75–25 without appreciably altering the Vmax upstroke kinetics (n > 550 cells per group). Box defines interquartile range; whiskers indicate ± 5th and 95th percentile; dots indicate outliers. Also note that siIP3R1 only minimally affected the Ca2+ kinetic parameters. siIP3R2 slowed Vmax upstroke and broadened the distribution of uptake phase kinetic parameters CaTD50 and CaTD75–25. miR-25 in contrast slowed the uptake phase parameters but did not appreciably affect Vmax upstroke (n > 550 cells per group). Box defines interquartile range; whiskers indicate ± 5th and 95th percentile; dots indicate outliers. Although IP3R1 might be a direct target of miR-25, several lines of evidence suggest that it is unlikely to mediate the effect of miR-25 in heart failure. IP3Rs are intracellular ligand-gated Ca2+ release channels41 that in the sarcoplasmic reticulum are associated with excitation–contraction coupling or spontaneous Ca2+ release and enhanced Ca2+ transients, whereas in the nuclear envelope they promote nuclear Ca2+ signalling42,43,44, but a specific role for IP3R1 in heart failure has not been identified. Nonetheless, the control of IP3R1 by miR-25 might have a critical role under conditions that sensitize cardiomyocytes to inositol-1,4,5-trisphosphate, such as in response to endothelin 1, angiotensin and phenylephrine23 or in local Ca2+ control24. Experiments were replicated two times.
Extended Data Figure 4 Effect of miR-25 and miR-92a overexpression in vivo.
a, Effect of AAV9-mediated cardiac gene transfer of miR-25 and miR-92a on target protein levels. Cardiac gene transfer of miR-25 and miR-92a increased levels of their respective miRNAs but miR-92a was selective against confirmed target integrin subunit α5 (ITGA5). b, c, Effects of AAV9-mediated cardiac gene transfer of miR-25 and miR-92a on cardiac function. There is a tendency towards decreased cardiac function in both ESPVR (b) and ΔP/Δtmax (c) in AAV9-miR-25-treated animals relative to control- (AAV9-VLP) and AAV9-miR-92a-treated animals. n = number of biological replicates (all included): n = 5 (AAV9-VLP); 4 (AAV9-miR-25); 5 (AAV9-miR-92a) for panels b, c. Data are represented as mean ± s.e.m. NS, not significant.
Extended Data Figure 5 AAV9-mediated gene transfer integration in ventricular myocardium.
The number of integrated miR-25 and miR-92a copies in ventricular myocardium 6 weeks after AAV-mediated gene transfer, determined by qPCR (see Methods). n = number of biological replicates (all included): n = 5 (AAV9-VLP), n = 4 (AAV9-miR-25) and n = 5 (AAV9-miR-92a). Data are represented as mean ± s.e.m. NS, not significant.
Extended Data Figure 6 Effects of anti-miR-25 on endogenous miR-25 and SERCA2a in wild-type and Serca2a-null hearts.
a–c, Anti-miR-25 or control (scrambled sequence) anti-miRNA was administered intravenously to sham-operated wild-type mice (Sham) or to unoperated Serca2a-cardiomyocyte null (S2a KO) mice38, as for the experiments in Fig. 4 (see Methods). a, Sham-operated animals were injected with anti-miRNAs 1 week after surgery and analysed at 4 weeks. S2a KO animals were injected with 4-OH tamoxifen intraperitoneally for 4 days to delete Serca2a (see Methods), injected with anti-miRNAs 1 week later and analysed 4 weeks later (5 weeks from initial 4-OH-tamoxifen injection). Note that anti-miR-25 decreased endogenous miR-25 levels in sham-operated wild-type and S2a KO mice relative to control-treated animals (n = 3). b, Furthermore, SERCA2a protein levels increased in the anti-miR-25 treated wild-type animals relative to control (n = 3). c, SERCA2a is absent in S2a KO hearts. WT, wild type. In all panels data are represented as mean ± s.e.m. NS, not significant. n = number of biological replicates (all included).
Extended Data Figure 7 Selectivity of anti-miR-25 on miR-25 family.
Expression levels of miR-25 and family members (miR-32, miR-92a and miR-92b) in sham-operated mice that had been injected with anti-miR-25 or control (scrambled sequence) anti-miRNA as in Fig. 4 (see Methods) (n = 3). n = number of biological replicates (all included). Note that control anti-miRNA in sham-operated animals did not alter expression of any of the miRNAs tested (blue bars). In contrast, anti-miR-25 significantly reduced levels of miR-25 but not other family members in sham-operated animals (red bars). Data are represented as mean ± s.e.m. NS, not significant.
Extended Data Figure 8 Effects of anti-miR-25 on cardiac function of WT and Serca2a-null hearts.
a–e, Anti-miR-25 or control (scrambled sequence) anti-miRNA was administered intravenously to sham-operated wild-type mice (Sham) or to unoperated Serca2a-cardiomyocyte null (S2a KO) mice38, as for the experiments in Fig. 4 (see Methods). Sham-operated animals were injected with anti-miRNAs 1 week after surgery and followed by echocardiography. S2a KO animals were generated by injection with 4-OH tamoxifen intraperitoneally for 4 days (see Methods) to delete Serca2a, then injected with anti-miRNAs 1 week later and followed by echocardiography. a, Representative two-dimensional guided M-mode images of the LVs from wild-type and S2a KO mice. b, c, Echocardiographic indices of left ventricular inner dimension during diastole, LVIDd (b), and systole, LVIDs (c) (n = 3 (sham plus control); 8, (sham plus anti-miR-25); 7 (S2a KO plus control); 11 (S2a-KO plus anti-miR-25) 4 weeks after control or anti-miRNA injection. d, Echocardiographic measurement of fractional shortening (FS) expressed as a percentage at time points after control or anti-miRNA injection (n = 4 (sham plus control); 3 (sham plus anti-miR-25); 3 (S2a KO plus control); 3 (S2a KO plus anti-miR-25)). S2a KO mice show characteristic dilation and decline in cardiac function after 4-OH tamoxifen-induced excision of Serca2a39. ***P < 0.001 (Student’s t-test for difference between S2a KO and sham-operated control anti-miRNA groups at week 4 after injection). e, Haemodynamic effect of anti-miR-25 and control anti-miRNA injection is represented by pressure–volume plots of treatment cohorts as indicated 4 weeks after injection of control or anti-miRNA. Note that specific anti-miR-25 and control anti-miRNA acted similarly in sham-operated wild-type animals (n = 3 (sham plus control); 3, (sham plus anti-miR-25); 2 (S2a KO plus control); 3 (S2a-KO plus anti-miR-25). Moreover, anti-miR-25 did not increase cardiac function of S2a KO mice, unlike TAC-operated wild-type mice (Fig. 4), suggesting that the beneficial effect on cardiac function depends on SERCA2a. Data are represented as mean ± s.e.m. n = number of biological replicates (all included). NS, not significant.
Extended Data Figure 9 Kaplan–Meier survival curve for anti-miR-25 treatment.
Survival probability is plotted over time, showing a cumulative protective effect of anti-miR-25 relative to control (scrambled sequence) anti-miRNA injections after TAC. The summary of two experiments is shown, plotting time from injection. Groups were sham-operated (n = 8), TAC plus anti-miR-25 (n = 8) and TAC plus control (scrambled sequence) anti-miRNA (n = 22). Note that injection with specific anti-miR-25 increased survival (P = 0.0131, log-rank test) relative to TAC plus control miRNA.
Extended Data Figure 10 Effect of anti-miR-25 on accumulation of SUMOylated SERCA2a.
Immunoblots of lysates from heart tissue at termination of the in vivo study shown in Fig. 4 (5.5 months after TAC, corresponding to 3.5 months after injection of anti-miR-25 or control (scrambled sequence) anti-miRNA). Lysates were immunoprecipitated with anti-SUMO1 followed by western blotting with anti-SERCA2a, as described previously21. Experiments were replicated three times. Note the reduction in SUMOylated SERCA2a upon TAC in the control anti-miRNA treated hearts, but restoration of expression after specific anti-miR-25 injection. Total levels of SUMO1 and actin are shown.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-3. (PDF 727 kb)
Rights and permissions
About this article
Cite this article
Wahlquist, C., Jeong, D., Rojas-Muñoz, A. et al. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature 508, 531–535 (2014). https://doi.org/10.1038/nature13073
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13073
This article is cited by
-
CVD phenotyping in oncologic disorders: cardio-miRNAs as a potential target to improve individual outcomes in revers cardio-oncology
Journal of Translational Medicine (2024)
-
The interplay of inflammation, exosomes and Ca2+ dynamics in diabetic cardiomyopathy
Cardiovascular Diabetology (2023)
-
Intestinal recruitment of CCR6-expressing Th17 cells by suppressing miR-681 alleviates endotoxemia-induced intestinal injury and reduces mortality
Inflammation Research (2023)
-
Non-Coding RNA-Mediated Gene Regulation in Cardiovascular Disorders: Current Insights and Future Directions
Journal of Cardiovascular Translational Research (2023)
-
Predictive biomarkers for the early detection and management of heart failure
Heart Failure Reviews (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.