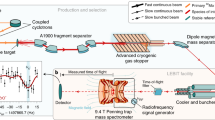
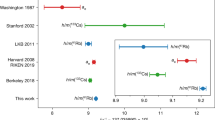
Abstract
The quest for the value of the electron’s atomic mass has been the subject of continuing efforts over the past few decades1,2,3,4. Among the seemingly fundamental constants that parameterize the Standard Model of physics5 and which are thus responsible for its predictive power, the electron mass me is prominent, being responsible for the structure and properties of atoms and molecules. It is closely linked to other fundamental constants, such as the Rydberg constant R∞ and the fine-structure constant α (ref. 6). However, the low mass of the electron considerably complicates its precise determination. Here we combine a very precise measurement of the magnetic moment of a single electron bound to a carbon nucleus with a state-of-the-art calculation in the framework of bound-state quantum electrodynamics. The precision of the resulting value for the atomic mass of the electron surpasses the current literature value of the Committee on Data for Science and Technology (CODATA6) by a factor of 13. This result lays the foundation for future fundamental physics experiments7,8 and precision tests of the Standard Model9,10,11.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Farnham, D. L., Van Dyck, R. S., Jr & Schwinberg, P. B. Determination of the electron's atomic mass and the proton/electron mass ratio. Phys. Rev. Lett. 75, 3598–3601 (1995)
Gräff, G., Kalinowsky, H. & Traut, J. A direct determination of the proton electron mass ratio. Z. Phys. A 297, 35–39 (1980)
Beier, T. et al. New determination of the electron’s mass. Phys. Rev. Lett. 88, 011603 (2001)
Hori, M. et al. Two-photon laser spectroscopy of antiprotonic helium and the antiproton-to-electron mass ratio. Nature 475, 484–488 (2011)
Cottingham, W. N. &. Greenwood, D. A. An Introduction to the Standard Model of Particle Physics (Cambridge Univ. Press, 2007)
Mohr, P. J., Taylor, B. N. & Newell, D. B. CODATA recommended values of the fundamental physical constants: 2010. Rev. Mod. Phys. 84, 1527–1605 (2012)
Shabaev, V. M. et al. g-Factor of heavy ions: a new access to the fine structure constant. Phys. Rev. Lett. 96, 253002 (2006)
Shabaev, V. M. et al. g-Factor of high-Z lithiumlike ions. Phys. Rev. A 65, 062104 (2002)
Hanneke, D., Fogwell, S. & Gabrielse, G. New measurement of the electron magnetic moment and the fine structure constant. Phys. Rev. Lett. 100, 120801 (2008)
Aoyama, T., Hayakawa, M., Kinoshita, T. & Nio, M. Tenth-order QED contribution to the electron g − 2 and an improved value of the fine structure constant. Phys. Rev. Lett. 109, 111807 (2012)
Bœhm, C. & Silk, J. A new test for dark matter particles of low mass. Phys. Lett. B 661, 287–289 (2008)
Häffner, H. et al. High-accuracy measurement of the magnetic moment anomaly of the electron. Phys. Rev. Lett. 85, 5308–5311 (2000)
Verdú, J. et al. Electronic g factor of hydrogenlike oxygen 16O7+. Phys. Rev. Lett. 92, 093002 (2004)
Pachucki, K., Czarnecki, A., Jentschura, U. D. & Yerokhin, V. A. Complete two-loop correction to the bound-electron g factor. Phys. Rev. A 72, 022108 (2005)
Breit, G. The magnetic moment of the electron. Nature 122, 649 (1928)
Beier, T. The g j factor of a bound electron and the hyperfine structure. Phys. Rep. 339, 79–213 (2000)
Yerokhin, V. A., Indelicato, P. & Shabaev, V. M. Evaluation of the self-energy correction to the g factor of S states in H-like ions. Phys. Rev. A 69, 052503 (2004)
Sturm, S. et al. g-factor measurement of hydrogenlike 28Si13+ as a challenge to QED calculations. Phys. Rev. A 87 (3),. 030501 (2013)
Sturm, S. et al. g Factor of hydrogenlike 28Si13+. Phys. Rev. Lett. 107, 023002 (2011)
Gabrielse, G. Why is sideband mass spectrometry possible with ions in a Penning trap? Phys. Rev. Lett. 102, 172501 (2009)
Sturm, S., Wagner, A., Schabinger, B. & Blaum, K. Phase-sensitive cyclotron frequency measurements at ultralow energies. Phys. Rev. Lett. 107, 143003 (2011)
Cornell, E. A. et al. Single-ion cyclotron resonance measurement of M(CO+)/M(N2+). Phys. Rev. Lett. 63, 1674–1677 (1989)
Dehmelt, H. Continuous Stern-Gerlach effect: principle and idealized apparatus. Proc. Natl Acad. Sci. USA 83, 2291–2294 (1986)
Brown, L. S. & Gabrielse, G. Geonium theory: physics of a single electron or ion in a Penning trap. Rev. Mod. Phys. 58, 233–311 (1986)
Kramida, A. Atomic Energy Levels and Spectra Bibliographic Database (version 2.0). http://physics.nist.gov/PhysRefData/ASD/ionEnergy.html (Physical Measurement Laboratory, Quantum Measurement Division, NIST)
Bouchendira, R., Cladé, P., Gouellati-Khélifa, S., Nez, F. & Biraben, F. New determination of the fine structure constant and test of the quantum electrodynamics. Phys. Rev. Lett. 106, 080801 (2011)
Mount, B. J., Redshaw, M. & Myers, E. G. Atomic masses of 6Li, 23Na, 39,41K, 85,87Rb and 133Cs. Phys. Rev. A 82, 042513 (2010)
Acknowledgements
This work was supported by the Max Planck Society, the EU (ERC grant number 290870; MEFUCO), the IMPRS-QD, GSI and the Helmholtz Alliance HA216/EMMI.
Author information
Authors and Affiliations
Contributions
S.S., F.K. and A.W. performed the experiment. S.S. and F.K. performed the data analysis. J.Z. performed the QED calculations. S.S., F.K., K.B., J.Z. and Z.H. prepared the manuscript. S.S. and F.K. prepared the experimental part of the Supplementary Information. J.Z. and Z.H. prepared the theoretical part of the Supplementary Information. All authors discussed the results and contributed to the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary text and Data, Supplementary Figures 1-2, Supplementary Tables 1-2 and Supplementary References. (PDF 494 kb)
Rights and permissions
About this article
Cite this article
Sturm, S., Köhler, F., Zatorski, J. et al. High-precision measurement of the atomic mass of the electron. Nature 506, 467–470 (2014). https://doi.org/10.1038/nature13026
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13026
This article is cited by
-
Stringent test of QED with hydrogen-like tin
Nature (2023)
-
Measurement of the bound-electron g-factor difference in coupled ions
Nature (2022)
-
Direct measurement of the 3He+ magnetic moments
Nature (2022)
-
Proton–electron mass ratio by high-resolution optical spectroscopy of ion ensembles in the resolved-carrier regime
Nature Physics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.