Abstract
A core promoter is a stretch of DNA surrounding the transcription start site (TSS) that integrates regulatory inputs1 and recruits general transcription factors to initiate transcription2. The nature and causative relationship of the DNA sequence and chromatin signals that govern the selection of most TSSs by RNA polymerase II remain unresolved. Maternal to zygotic transition represents the most marked change of the transcriptome repertoire in the vertebrate life cycle3,4,5,6. Early embryonic development in zebrafish is characterized by a series of transcriptionally silent cell cycles regulated by inherited maternal gene products: zygotic genome activation commences at the tenth cell cycle, marking the mid-blastula transition7. This transition provides a unique opportunity to study the rules of TSS selection and the hierarchy of events linking transcription initiation with key chromatin modifications. We analysed TSS usage during zebrafish early embryonic development at high resolution using cap analysis of gene expression8, and determined the positions of H3K4me3-marked promoter-associated nucleosomes9. Here we show that the transition from the maternal to zygotic transcriptome is characterized by a switch between two fundamentally different modes of defining transcription initiation, which drive the dynamic change of TSS usage and promoter shape. A maternal-specific TSS selection, which requires an A/T-rich (W-box) motif, is replaced with a zygotic TSS selection grammar characterized by broader patterns of dinucleotide enrichments, precisely aligned with the first downstream (+1) nucleosome. The developmental dynamics of the H3K4me3-marked nucleosomes reveal their DNA-sequence-associated positioning at promoters before zygotic transcription and subsequent transcription-independent adjustment to the final position downstream of the zygotic TSS. The two TSS-defining grammars coexist, often physically overlapping, in core promoters of constitutively expressed genes to enable their expression in the two regulatory environments. The dissection of overlapping core promoter determinants represents a framework for future studies of promoter structure and function across different regulatory contexts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
19 March 2014
A change was made to the Acknowledgements section.
References
D’Alessio, J. A., Wright, K. J. & Tjian, R. Shifting players and paradigms in cell-specific transcription. Mol. Cell 36, 924–931 (2009)
Kadonaga, J. T. Perspectives on the RNA polymerase II core promoter. Wiley Interdiscip. Rev. Dev. Biol. 1, 40–51 (2012)
Mathavan, S. et al. Transcriptome analysis of zebrafish embryogenesis using microarrays. PLoS Genet. 1, e29 (2005)
Tadros, W. & Lipshitz, H. D. The maternal-to-zygotic transition: a play in two acts. Development 136, 3033–3042 (2009)
Vastenhouw, N. L. et al. Chromatin signature of embryonic pluripotency is established during genome activation. Nature 464, 922–926 (2010)
Lindeman, L. C. et al. Prepatterning of developmental gene expression by modified histones before zygotic genome activation. Dev. Cell 21, 993–1004 (2011)
Kane, D. A. & Kimmel, C. B. The zebrafish midblastula transition. Development 119, 447–456 (1993)
Shiraki, T. et al. Cap analysis gene expression for high-throughput analysis of transcriptional starting point and identification of promoter usage. Proc. Natl Acad. Sci. USA 100, 15776–15781 (2003)
Barski, A. et al. High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837 (2007)
Carninci, P. et al. Genome-wide analysis of mammalian promoter architecture and evolution. Nature Genet. 38, 626–635 (2006)
Lenhard, B., Sandelin, A. & Carninci, P. Metazoan promoters: emerging characteristics and insights into transcriptional regulation. Nature Rev. Genet. 13, 233–245 (2012)
Venters, B. J. & Pugh, B. F. Genomic organization of human transcription initiation complexes. Nature 502, 53–58 (2013)
Nepal, C. et al. Dynamic regulation of the transcription initiation landscape at single nucleotide resolution during vertebrate embryogenesis. Genome Res. 23, 1938–1950 (2013)
Giraldez, A. J. et al. Zebrafish miR-430 promotes deadenylation and clearance of maternal mRNAs. Science 312, 75–79 (2006)
Ponjavic, J. et al. Transcriptional and structural impact of TATA-initiation site spacing in mammalian core promoters. Genome Biol. 7, R78 (2006)
Segal, E. et al. A genomic code for nucleosome positioning. Nature 442, 772–778 (2006)
Ioshikhes, I., Hosid, S. & Pugh, B. F. Variety of genomic DNA patterns for nucleosome positioning. Genome Res. 21, 1863–1871 (2011)
Segal, E. & Widom, J. What controls nucleosome positions? Trends Genet. 25, 335–343 (2009)
Rhee, H. S. & Pugh, B. F. Genome-wide structure and organization of eukaryotic pre-initiation complexes. Nature 483, 295–301 (2012)
Rach, E. A. et al. Transcription initiation patterns indicate divergent strategies for gene regulation at the chromatin level. PLoS Genet. 7, e1001274 (2011)
Cairns, B. R. The logic of chromatin architecture and remodelling at promoters. Nature 461, 193–198 (2009)
Deaton, A. M. & Bird, A. CpG islands and the regulation of transcription. Genes Dev. 25, 1010–1022 (2011)
Thomson, J. P. et al. CpG islands influence chromatin structure via the CpG-binding protein Cfp1. Nature 464, 1082–1086 (2010)
Fenouil, R. et al. CpG islands and GC content dictate nucleosome depletion in a transcription-independent manner at mammalian promoters. Genome Res. 22, 2399–2408 (2012)
Nozaki, T. et al. Tight associations between transcription promoter type and epigenetic variation in histone positioning and modification. BMC Genomics 12, 416 (2011)
Bártfai, R. et al. TBP2, a vertebrate-specific member of the TBP family, is required in embryonic development of zebrafish. Curr. Biol. 14, 593–598 (2004)
Akhtar, W. & Veenstra, G. TBP2 is a substitute for TBP in Xenopus oocyte transcription. BMC Biol. 7, 45 (2009)
Lauberth, S. M. et al. H3K4me3 interactions with TAF3 regulate preinitiation complex assembly and selective gene activation. Cell 152, 1021–1036 (2013)
Zaugg, J. B. & Luscombe, N. M. A genomic model of condition-specific nucleosome behavior explains transcriptional activity in yeast. Genome Res. 22, 84–94 (2012)
Davis, W., Jr & Schultz, R. M. Developmental change in TATA-box utilization during preimplantation mouse development. Dev. Biol. 218, 275–283 (2000)
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009)
Kodzius, R. et al. CAGE: cap analysis of gene expression. Nature Methods 3, 211–222 (2006)
Balwierz, P. J. et al. Methods for analyzing deep sequencing expression data: constructing the human and mouse promoterome with deepCAGE data. Genome Biol. 10, R79 (2009)
The R Development Core Team. R: A Language and Environment for Statistical Computing 1–3079 (R Foundation for Statistical Computing, 2013)
Gentleman, R. C. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80 (2004)
Törönen, P., Kolehmainen, M., Wong, G. & Castren, E. Analysis of gene expression data using self-organizing maps. FEBS Lett. 451, 142–146 (1999)
Portales-Casamar, E. et al. JASPAR 2010: the greatly expanded open-access database of transcription factor binding profiles. Nucleic Acids Res. 38, D105–D110 (2010)
Bailey, T. L. & Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Sec. Int. Conf. Intell. Syst. Mol. Biol. 2, 28–36 (1994)
Abe, G., Suster, M. L. & Kawakami, K. in The Zebrafish: Genetics, Genomics and Informatics (eds Detrich, H. W., Westerfield, M. & Zon, L. I. ) Vol. 104, 23–49 (Academic, 2011)
Gehrig, J. et al. Automated high-throughput mapping of promoter-enhancer interactions in zebrafish embryos. Nature Methods 6, 911–916 (2009)
Higashijima, S., Okamoto, H., Ueno, N., Hotta, Y. & Eguchi, G. High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Dev. Biol. 192, 289–299 (1997)
Ferg, M. Large Scale- and Functional Analysis for the Requirement of TBP-Function in Early Zebrafish Development. PhD thesis, Heidelberg Univ. (2008)
Ferg, M. et al. The TATA-binding protein regulates maternal mRNA degradation and differential zygotic transcription in zebrafish. EMBO J. 26, 3945–3956 (2007)
Soler, E. et al. A systems approach to analyze transcription factors in mammalian cells. Methods 53, 151–162 (2011)
Zhang, Y. et al. Model-based Analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008)
Acknowledgements
The authors are grateful to L. Tora, E. Kenyon, G. Chalancon and J. C.-y. Chen for comments on the manuscript, to M. Reischl for Zebrafish Miner software, and to L. O’Neill for technical advice. V.H., C.N., C.Pr., A.A. and X.D. were supported by grants from the Norwegian Research Council (YFF) and the Bergen Research Foundation, awarded to B.L. F.M., U.S. and B.L. acknowledge support from European Union (EU) FP6 integrated project EuTRACC and FP7 integrated project ZF Health. B.L. was additionally supported by the Medical Research Council UK, F.M. and P.C. by the EU FP7 project Dopaminet and U.S. by the EU FP6 project NeuroXSys. C.Pl., A.M.S. and P.C. were supported by a Research Grant from MEXT to RIKEN CLST. (RIKEN Omics Science Center ceased to exist on 1 April 2013 owing to reorganization of RIKEN.)
Author information
Authors and Affiliations
Contributions
B.L., F.M., P.C. and V.H. conceived the study. N.L., Y.H., J.G. and M.F. performed experiments. O.A. and W.F.J.v.I. performed sequencing. V.H., C.Pr., C.N., X.D. and A.A. performed computational analyses. C.Pl. and A.M.S. developed and performed SL-CAGE with input from P.C. V.H., B.L. and F.M. analysed the data and wrote the manuscript with input from P.C., W.F.J.v.I. and U.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
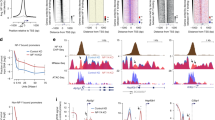
Extended Data Figure 1 Promoters contain TSSs with very heterogeneous developmental expression profiles.
a, Developmental expression profiles of CAGE-defined promoters obtained by self-organizing map (SOM) clustering. Each box represents one SOM cluster, with a series of beanplots showing distribution of scaled expression (logarithm of normalized number of CAGE tags per million) at different time points for all promoters belonging to that cluster (number of promoters denoted above the box). Different classes of promoters marked in different colours were used in all downstream analyses. b, Projection of maternal- and zygotic-specific CTSSs (from Fig. 1b) onto the SOM classes of promoters shown in a. c, Fraction of promoters containing either maternal-specific (cluster 4,4 in Fig. 1b) or zygotic-specific (cluster 0,0 in Fig. 1b) CTSS, or both, for different classes of promoters established in a. d, Example of a throughout-active promoter that contains a very heterogeneous population of CTSSs (colour coding of CTSSs as in Fig. 1b: maternal-specific shown in blue, throughout-expressed shown in green, and zygotic-specific shown in red).
Extended Data Figure 2 Definition and properties of shifting promoters.
a, Schematic representation of shifting promoter score calculation between two stages. Forward and reverse scores are calculated using forward (F1, F2) and reverse (F1′, F2′) cumulative sums of CAGE tags along the promoter region, and the final shifting score is the maximum of the two. b, Heatmap showing the number of shifting promoters detected by applying the method shown in a (using a 0.6 score threshold) to all possible pairs of developmental stages. The majority of shifting was detected between the first six stages (predominantly maternal) and last three stages (predominantly zygotic). Promoters that qualified as shifting in at least four of these early versus late pairs were selected for a final set of 911 shifting promoters. c, Distribution of distance between maternal and zygotic dominant TSS (shift) for 911 shifting promoters. d, Developmental expression dynamics of maternal (blue) and zygotic (red) dominant TSS in shifting promoters. Average expression for the entire promoter is shown in black. e, Density of AA, AT, TT and CG dinucleotides for shifting promoters sorted by magnitude of shift. Promoters were centred at either the maternal (left) or the zygotic (right) dominant TSS. Blue arrowhead marks the AA/AT/TT enrichment ∼30 bp upstream of the maternal TSS and red arrowhead marks the boundary between CG and AA/AT/TT enrichment ∼50 bp downstream of the zygotic TSS. f, Relative frequency of top ten most frequent TATA-like pentamers in the region −35 to −22 bp upstream of maternal (blue) and zygotic (red) dominant TSS. Percentage of match to TATA-box PWM is denoted in the brackets.
Extended Data Figure 3 Genome-wide sequence and chromatin signature of maternal versus zygotic TSSs.
a, Dinucleotide density for all throughout-active promoters sorted by orientation-sensitive distance between the maternal and zygotic dominant TSS. Promoters were centred at either the maternal (left) or the zygotic (right) dominant TSS. b, Distribution of match (%) to TATA-box PWM in the region −35 to −22 bp upstream of the maternal (blue) and zygotic (red) dominant TSS in constitutively active promoters. P value of the two-tailed Wilcoxon rank-sum test is shown. c, Relative frequency of top ten most frequent TATA-like pentamers upstream of the maternal (blue) and zygotic (red) dominant TSS. Percentage of match to TATA-box PWM is denoted in the brackets. d, H2A.Z signal in the prim 6 stage at the same set of promoters as in a. Subtracted coverage of reads mapping to the plus and minus strand is shown. e, Density of TT dinucleotide in a ±100 bp region for the same set of promoters as in a. f, WW dinucleotide density for maternal- (left) and zygotic-specific (right) promoters centred at their dominant TSS and sorted by decreasing match to the TATA-box (white dashed lines). Average density of individual dinucleotides is shown below. g, Distribution of match (%) to TATA-box PWM in the region –35 to –22 bp upstream of dominant TSS for maternal- (blue) and zygotic-specific (red) promoters. P value of the two-tailed Wilcoxon rank-sum test is shown. h, Relative frequency of the top ten most abundant TATA-like pentamers in maternal (blue) and zygotic (red) promoters. Percentage of match to TATA-box PWM is denoted in the brackets.
Extended Data Figure 4 Composite multiple sharp architecture of maternal-specific promoters.
a, Distribution of promoter interquantile width (q0.1 − q0.9; central promoter region that contains ≥80% of CAGE tags) for 1,944 maternal-specific promoters. b, Number of TCs within the promoter region for maternal broad promoters (interquantile width >20 bp) across early developmental stages. Bubble size reflects relative number of promoters that contain given number of TCs. c, Comparison of dinucleotide frequencies around single dominant TSS in promoter (left) and dominant TSSs from all TCs within the same promoter (right). d, Composite architecture of a maternal broad promoter showing multiple sharp TCs with their associated W-box at the expected ∼30 bp upstream position.
Extended Data Figure 5 Change of promoter architecture during maternal to zygotic transition.
a, Examples of maternal-specific, zygotic-specific and throughout-active promoters showing difference in architecture between maternal and zygotic stage. b, Number of TCs within promoter region for maternal-specific, zygotic-specific and constitutively active promoters. Bubble size reflects relative number of promoters that contain given number of TCs and different colours correspond to different developmental stages. c, Distribution of distance between neighbouring TCs within the same promoter region. d, Heatmap showing k-means clustering (k = 15) of throughout-active promoters, based on the number of TCs that they contain throughout 12 developmental stages. e, Representative examples for three selected clusters: highly expressed sharp promoter whose architecture does not change and retains only one TC (left); promoter in which number of TCs decreases from five clearly separated sharp TCs in maternal stages to one fairly broad TC in zygotic stages (middle); promoter that changes its architecture from two clearly separated sharp TCs to one broader TC after maternal to zygotic transition (right).
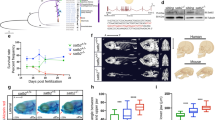
Extended Data Figure 6 Validation of sf3a2 promoter code in transgene injected embryos.
a, Schematic of reporter construct; the dashed lines indicate the spliced out intronic sequence. SA, splice acceptor; SD, splice donor. b, Primers used for sf3a2 construct. c, Reporter gene expression in the wild-type and mutant sf3a2 construct-injected embryos (36 hpf). Bright field (BF) and red channel fluorescent images (mCherry) are shown. Scale bar, 250 μm. The panels on the right show red channel projection overlay of 24 (wild type (WT), top) and 31 (mutant (mut), bottom) embryos. d, Percentage of mCherry-expressing embryos from total ECFP-positive embryos (left) and overall reporter activity measured as average pixel intensity values from 24 wild-type and 31 mutant sf3a2 promoter-injected embryos (right). e, 5′ RACE of sf3a2 promoter construct. Fragments amplified by PCR using a forward primer specific for the 5′ RACE adaptor and two different inner reverse primers specific for the mCherry reporter. The arrowheads indicate the PCR products of expected size. A, 5′ RACE adaptor; IF, inner forward primer; IR1, inner reverse primer 1; IR2, inner reverse primer 2; M, DNA marker; mut, embryos injected with construct containing sf3a2 promoter with mutations disrupting W-boxes (as shown in f); TAP, tobacco acid pyrophosphatase; WT, embryos injected with construct containing wild-type sf3a2 promoter. f, Primers used in 5′ RACE. g, Genomic sequence of the sf3a2 promoter with point mutations introduced to disrupt W-boxes (orange) marked in red. The track at the bottom shows TSSs detected by CAGE in wild-type zebrafish embryos in prim 6 zygotic stage. Black arrow marks TSS confirmed by 5′ RACE in the zygotic stage in both the wild-type and the mutated sf3a2 promoter.
Extended Data Figure 7 Validation of sf3a2 promoter code in stable transgenic lines.
a, sf3a2:mCherry transgene expression in wild-type (WT; top four panels) and mutant (MUT; bottom four panels) sf3a2 promoter transgenic embryos (high stage). Scale bar, 1 mm. b, Quantitative polymerase chain reaction (qPCR) results of transgene expression: ratio between abundance of sf3a2:mCherry trasgene mRNA and endogenous sf3a2 mRNA. Bar height shows mean value across six qPCR experiments (three replicas for each of the two different primer pairs) and error bars denote 95% confidence interval of the mean. c, Sequence of primers used in qPCR. d, TSSs and their relative usage detected by SL-CAGE in three wild-type and four mutant sf3a2 promoter transgenic lines. Promoter sequence is shown at the top, with point mutations introduced to disrupt W-boxes highlighted in red. For each transgenic line two tracks are shown: TSSs used by the endogenous sf3a2 promoter (upper track, more intensive colour) and TSSs used by the transgene (lower track, lighter colour). P value (one-tailed Welch’s two sample t-test) of the difference between mutated and wild-type promoter variants is shown for four maternal TSSs associated with disrupted W-boxes (dashed boxes).
Extended Data Figure 8 H3K4me3-marked nucleosome positioning signal in maternal and zygotic stage.
a, Venn diagrams showing number of promoters containing H3K4me3 peak within ±1 kb of TSS in early (512 cells) and late (prim 6) stage for distinct classes of promoters (as defined in Extended Data Fig. 1a). b, Frequency of selected dinucleotides centred at the position of the first downstream (+1) nucleosome for distinct classes of promoters. Only promoters containing an H3K4me3 peak in both 512 cells and prim 6 stages were used. Centres of nucleosomes were estimated from subtracted H3K4me3 coverage (shown in grey). Density of TSSs in maternal (512 cells) and zygotic (prim 6) stages is shown in light blue and light red, respectively. Note that maternal-specific promoters have only maternal TSSs and the distribution of these TSSs in the zygotic stage is shown only for orientation. The opposite is true for zygotic-specific promoters.
Extended Data Figure 9 Validation of Tbp morphant phenotypes before ChIP-seq analysis.
Tbp morphants show epiboly defects and differential loss of reporter activity as previously described42,43. Embryos in the 30% epiboly stage showing epiboly delay and corresponding Tbp-independent (ntl:yfp) and Tbp-dependent (β-actin:yfp) reporter gene activities were used for H3K4me3 ChIP-seq analysis. Zebrafish fertilized eggs were injected with the reporter constructs as indicated on the right followed by injection of morpholino (Tbp 5 mismatch or Tbp mo1 morpholino42, respectively). Fluorescence images on top, bright field images below. ntl, notail; yfp, yellow fluorescent protein. Scale bar, 400 μm.
Extended Data Figure 10 Dinucleotide maps: calculation and visualization of dinucleotide patterns.
Genomic sequences (of the same length) are sorted and aligned into a matrix-like representation (P1–P10, positions; S1–S10, sequences). Marking the presence of selected dinucleotide (for example, AA) by 1 and the absence by 0 creates an occurrence matrix. Next, a weighted average is calculated at each position by placing a two-dimensional Gaussian kernel at that position and assigning weights to surrounding positions. An example of calculating the value at position S4,P7 is shown. Surrounding positions are coloured on the basis of the weights assigned to them by the Gaussian kernel (bandwidth = 1 in both dimensions, and covariance = 0 between the two dimensions). Averaged values are mapped to different shades of blue to visualize the dinucleotide density across the set of input sequences.
Supplementary information
Supplementary Table 1
This file contains a list of 911 shifting promoters with shifting scores, positions of maternal and zygotic dominant TSS and associated annotated gene. (XLS 244 kb)
Supplementary Table 2
This file contains single locus deep CAGE (SL-CAGE) steps and sequences of used primers. (XLS 39 kb)
Rights and permissions
About this article
Cite this article
Haberle, V., Li, N., Hadzhiev, Y. et al. Two independent transcription initiation codes overlap on vertebrate core promoters. Nature 507, 381–385 (2014). https://doi.org/10.1038/nature12974
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12974
This article is cited by
-
High-throughput analysis of the Trypanosoma cruzi minicirculome (mcDNA) unveils structural variation and functional diversity
Scientific Reports (2024)
-
Energy-driven genome regulation by ATP-dependent chromatin remodellers
Nature Reviews Molecular Cell Biology (2024)
-
Alternative promoters in CpG depleted regions are prevalently associated with epigenetic misregulation of liver cancer transcriptomes
Nature Communications (2023)
-
Transposable element-initiated enhancer-like elements generate the subgenome-biased spike specificity of polyploid wheat
Nature Communications (2023)
-
Establishment and function of chromatin organization at replication origins
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.