Abstract
The transcription factors c-Myc and N-Myc—encoded by Myc and Mycn, respectively—regulate cellular growth1 and are required for embryonic development2,3. A third paralogue, Mycl1, is dispensable for normal embryonic development but its biological function has remained unclear4. To examine the in vivo function of Mycl1 in mice, we generated an inactivating Mycl1gfp allele that also reports Mycl1 expression. We find that Mycl1 is selectively expressed in dendritic cells (DCs) of the immune system and controlled by IRF8, and that during DC development, Mycl1 expression is initiated in the common DC progenitor5 concurrent with reduction in c-Myc expression. Mature DCs lack expression of c-Myc and N-Myc but maintain L-Myc expression even in the presence of inflammatory signals such as granulocyte–macrophage colony-stimulating factor. All DC subsets develop in Mycl1-deficient mice, but some subsets such as migratory CD103+ conventional DCs in the lung and liver are greatly reduced at steady state. Importantly, loss of L-Myc by DCs causes a significant decrease in in vivo T-cell priming during infection by Listeria monocytogenes and vesicular stomatitis virus. The replacement of c-Myc by L-Myc in immature DCs may provide for Myc transcriptional activity in the setting of inflammation that is required for optimal T-cell priming6.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dang, C. V. MYC on the path to cancer. Cell 149, 22–35 (2012)
Charron, J. et al. Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 6, 2248–2257 (1992)
Davis, A. C., Wims, M., Spotts, G. D., Hann, S. R. & Bradley, A. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 7, 671–682 (1993)
Hatton, K. S. et al. Expression and activity of L-Myc in normal mouse development. Mol. Cell. Biol. 16, 1794–1804 (1996)
Liu, K. et al. In vivo analysis of dendritic cell development and homeostasis. Science 324, 392–397 (2009)
Lauvau, G. et al. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science 294, 1735–1739 (2001)
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011)
Laurenti, E. et al. Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell 3, 611–624 (2008)
Rahl, P. B. et al. c-Myc regulates transcriptional pause release. Cell 141, 432–445 (2010)
Lin, C. Y. et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 151, 56–67 (2012)
Nie, Z. et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell 151, 68–79 (2012)
Wasylishen, A. R. et al. New model systems provide insights into Myc-induced transformation. Oncogene 30, 3727–3734 (2011)
Nakagawa, M., Takizawa, N., Narita, M., Ichisaka, T. & Yamanaka, S. Promotion of direct reprogramming by transformation-deficient Myc. Proc. Natl Acad. Sci. USA 107, 14152–14157 (2010)
Kabashima, K. et al. Intrinsic lymphotoxin-β receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity 22, 439–450 (2005)
Liu, K. et al. Origin of dendritic cells in peripheral lymphoid organs of mice. Nature Immunol. 8, 578–583 (2007)
Huang, C. Y., Bredemeyer, A. L., Walker, L. M., Bassing, C. H. & Sleckman, B. P. Dynamic regulation of c-Myc proto-oncogene expression during lymphocyte development revealed by a GFP-c-Myc knock-in mouse. Eur. J. Immunol. 38, 342–349 (2008)
Satpathy, A. T. et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J. Exp. Med. 209, 1135–1152 (2012)
Becker, A. M. et al. IRF-8 extinguishes neutrophil production and promotes dendritic cell lineage commitment in both myeloid and lymphoid mouse progenitors. Blood 119, 2003–2012 (2012)
Tailor, P., Tamura, T., Morse, H. C. & Ozato, K. The BXH2 mutation in IRF8 differentially impairs dendritic cell subset development in the mouse. Blood 111, 1942–1945 (2008)
Inaba, K. et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693–1702 (1992)
Tussiwand, R. et al. Compensatory dendritic cell development mediated by BATF–IRF interactions. Nature 490, 502–507 (2012)
Scott, C. L. et al. Functional analysis of mature hematopoietic cells from mice lacking the βc chain of the granulocyte-macrophage colony-stimulating factor receptor. Blood 92, 4119–4127 (1998)
Shortman, K. & Heath, W. R. The CD8+ dendritic cell subset. Immunol. Rev. 234, 18–31 (2010)
Hildner, K. et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008)
Swiecki, M., Gilfillan, S., Vermi, W., Wang, Y. & Colonna, M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8+ T cell accrual. Immunity 33, 955–966 (2010)
Satpathy, A. T. et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nature Immunol. 14, 937–948 (2013)
Meredith, M. M. et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J. Exp. Med. 209, 1153–1165 (2012)
Edelson, B. T. et al. CD8α+ dendritic cells are an obligate cellular entry point for productive infection by Listeria monocytogenes. Immunity 35, 236–248 (2011)
Neuenhahn, M. et al. CD8α+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity 25, 619–630 (2006)
Resnitzky, D. & Kimchi, A. Deregulated c-myc expression abrogates the interferon- and interleukin 6-mediated G0/G1 cell cycle arrest but not other inhibitory responses in M1 myeloblastic cells. Cell Growth Differ. 2, 33–41 (1991)
Iiizumi, S. et al. Simple one-week method to construct gene-targeting vectors: application to production of human knockout cell lines. Biotechniques 41, 311–316 (2006)
Dignam, J. D., Lebovitz, R. M. & Roeder, R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11, 1475–1489 (1983)
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010)
Pope, C. et al. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166, 3402–3409 (2001)
Kim, S. K. et al. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc. Natl Acad. Sci. USA 95, 10814–10819 (1998)
Verschoor, A. et al. A platelet-mediated system for shuttling blood-borne bacteria to CD8α+ dendritic cells depends on glycoprotein GPIb and complement C3. Nature Immunol. 12, 1194–1201 (2011)
Belz, G. T. Direct ex vivo activation of T cells for analysis of dendritic cells antigen presentation. Methods Mol. Biol. 595, 351–369 (2010)
Lee, T. I., Johnstone, S. E. & Young, R. A. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nature Protocols 1, 729–748 (2006)
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008)
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010)
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005)
Huang, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols 4, 44–57 (2008)
Edelson, B. T. et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 207, 823–836 (2010)
Ginhoux, F. et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 204, 3133–3146 (2007)
Lefrancois, L. & Lycke, N. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer’s patch, and lamina propria cells. Curr. Protoc. Immunol. 3, Unit–3.19 (2001)
Murphy, D. J. et al. Distinct thresholds govern Myc’s biological output in vivo. Cancer Cell 14, 447–457 (2008)
Feil, R., Wagner, J., Metzger, D. & Chambon, P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun. 237, 752–757 (1997)
Mahfoudi, A., Roulet, E., Dauvois, S., Parker, M. G. & Wahli, W. Specific mutations in the estrogen receptor change the properties of antiestrogens to full agonists. Proc. Natl Acad. Sci. USA 92, 4206–4210 (1995)
Sedy, J. R. et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nature Immunol. 6, 90–98 (2005)
Acknowledgements
This work was supported by the Howard Hughes Medical Institute and the Siteman Cancer Center (K.M.M.), American Heart Association (12PRE8610005 to A.T.S. and 12PRE12050419 to W.K.), German Research Foundation (AL 1038/1-1; J.C.A.), American Society of Hematology Scholar Award and Burroughs Wellcome Fund Career Award for Medical Scientists (B.T.E.). We thank J. M. White for blastocyst injections and generation of mouse chimaeras, the Alvin J. Siteman Cancer Center for use of the Center for Biomedical Informatics and the Multiplexed Gene Analysis Core Facility. The Siteman Cancer Center is supported in part by the NCI Cancer Center Support Grant P30 CA91842.
Author information
Authors and Affiliations
Contributions
W.K., T.L.M. and K.M.M. directed the work and wrote the manuscript. S.P.P. and P.M.A. helped with L. monocytogenes infections. A.S.R., M. Cella and M. Colonna helped with VSV infections. B.P.S. provided mice expressing GFP–c-Myc. J.L. and M.C.N. provided Zbtb46dtr/dtr mice. A.T.S. and J.C.A. aided gene targeting and analysis of DC populations. C.G.B., X.W., B.T.E., V.D., N.M.K. and E.V.R.-G. aided analysis of infections and histological analysis. X.W. aided microarray analysis. All authors discussed the results and contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Mycl1 expression within the immune system is restricted to DCs.
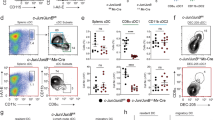
a, Shown is the percentage of BrdU+ cells within each indicated lineage from wild-type (WT) mice 4 h after BrdU administration. Data are from 2 independent experiments (error bars, ± s.d.; n = 9 mice per group, one-way analysis of variance (ANOVA) Tukey’s post-hoc test). b, Shown are single-colour histograms of DAPI and Ki-67 expression for indicated cell types from the spleen of wild-type mice. Dotted lines represent isotype control staining. Numbers represent percentage of cells in the indicated gate. Data are representative of 3 independent experiments. c, Shown are two-colour histograms of CD11c and SiglecH expression for all live bone marrow cells from wild-type mice, or B220 and MHC-II expression for CD11c+SiglecH+ cells, which were evaluated for DAPI expression (bottom panels). d, Relative Mycl1 messenger RNA expression was determined by quantitative PCR (qPCR) in pDCs isolated from bone marrow and other indicated cell types isolated from the spleen of wild-type mice. Shown is a graph of Mycl1 values normalized to Hprt values (error bars, s.d.; n = 3 biological replicates per cell type). e, CMP and CDP populations described in Methods were analysed by gene expression microarray. Shown are transcription factor probe sets with normalized linear expression value in the CDP greater than 100. The horizontal axis indicates the ratio of gene expression in the CMP compared to the CDP, and the vertical axis indicates the ratio of the mean expression in CD8α+ cDCs, CD11b+ cDCs and pDCs compared to a tissue panel excluding DCs (described in Methods). f, Relative Mycl1 mRNA expression was determined by qPCR for indicated mice. Shown is a graph of Mycl1 values normalized to Hprt values (error bars, s.d.; n = 3 biological replicates per cell type). g, Splenocytes from wild-type and Mycl1gfp/gfp mice on the C57BL/6 genetic background were stained for analysis, and pDCs (CD11cintSiglecH+), CD8α+ DCs (CD11c+MHC-II+CD8α+CD205+SiglecH−) and CD8α− DCs (CD11c+MHC-II+CD8α−CD205−SiglecH−) were identified. Shown are two-colour histograms of CD11c and SiglecH expression, MHC-II and CD11c expression, and CD8α and CD205 expression for pre-gated cells as indicated above the plots. Numbers represent percentage of cells in the indicated gate. Data are representative of at least 5 independent experiments. h, Relative Myc mRNA expression was determined by qPCR for indicated mice. Shown is a graph of Myc values normalized to Hprt values (error bars, ± s.d.; n = 3 biological replicates per cell type, Student’s t-test). *P < 0.05; NS, P > 0.05; ND, not detected.
Extended Data Figure 2 Generation of Mycl1gfp/gfp mice by homologous recombination.
a, The genomic context for Mycl1, the targeting vector, and targeted allele are shown. NotI restriction digest of the wild-type locus generates a 12.5-kilobase (kb) fragment detectable by either 5′ or 3′ probes. NcoI restriction digest of the targeted locus generates a 3.3-kb fragment detected by the 5′ probe, a 2.3-kb fragment containing most of the GFP.Neo cassette, and a 9.3-kb fragment detected by the 3′ probe. b, c, Southern blot analysis of NcoI-digested total DNA using the 5′ probe (b) and 3′ probe (c). Shown are results obtained from genomic DNA of wild-type (Mycl1+/+) and heterozygous (Mycl1+/gfp) mice. d, Genotyping PCR using genomic DNA of wild-type (Mycl1+/+), heterozygous (Mycl1+/gfp) and homozygous (Mycl1gfp/gfp) mice. The wild-type allele yields a 337-base pair (bp) product and the targeted allele yields a 525-bp product.
Extended Data Figure 3 Mycl1 expression is restricted to DCs in lymphoid and non-lymphoid tissues.
a, Shown are single-colour histograms of GFP expression for the indicated cells derived from wild-type, Mycl1+/gfp and Mycl1gfp/gfp bone marrow cells cultured in Flt3L for 10 days. cDCs (Flt3L cDC) were gated as CD11c+MHCII+SiglecH− and pDCs (Flt3L pDC) were gated as CD11cintSiglecH+. Numbers represent GFP+ cells as a percentage of live cells. Data are representative of 3 independent experiments. b, Shown are two-colour histograms of CD24 and SIRP-α expression for Flt3L cDCs as described in panel a, from bone marrow of wild-type mice (left panel) and Mycl1+/gfp mice (middle and right panels). Flt3L cDCs from bone marrow of Mycl1+/gfp mice were pre-gated as either GFP+ or GFP−. Numbers represent the percentage of cells in the indicated gate. c, Flt3+ CMPs purified by cell sorting from wild-type bone marrow cells were cultured in Flt3L for 12 h before transduction with control (empty) retrovirus or with retrovirus expressing IRF8, L-Myc or c-Myc. Cells were cultured for an additional 5 days and then stained for analysis. Shown is a two-colour histogram of MHC-II and SiglecH expression for each indicated transduction. cDCs are gated as MHC-II+SiglecH−and pDCs are gated as MHC-II−SiglecH+. Numbers represent the percentage of cells in the quadrant gate. d, Shown is a quantification of undifferentiated (non-cDC or non-pDC) cells from c. Data are from 4 independent transductions per retrovirus (error bars, s.d.; n = 4, one-way ANOVA Tukey’s post-hoc test). e, Cells from the spleen (left panel), inguinal lymph nodes (ILN, middle panel) and mesenteric lymph nodes (MLN, right panel) of wild-type, Mycgfp/gfp and Mycl1+/gfp mice were stained for analysis. Shown are two-colour histograms of CD11c and GFP expression for non-auto-fluorescent cells. Numbers represent percentage of cells in the indicated gate. Data are representative of at least 5 independent experiments. f, Shown are single-colour histograms of GFP expression for resident DCs (CD11c+MHC-IIint; DC) and migratory DCs (CD11cintMHC-II+; mDC) from mesenteric lymph nodes (top panel) and inguinal lymph nodes (bottom panel) of wild-type (grey lines) and Mycl1+/gfp (green lines) mice. Resident DCs were further gated as CD24+SIRP-α− (CD8α+ DC) and CD24−SIRP-α+ (CD8α− DC). Mesenteric lymph node mDCs were gated as CD103+CD11b− (CD103+ mDC), CD103+CD11b+ (CD103+CD11b+ mDC) and CD103−CD11b+ (CD11b+ mDC). ILN mDCs were gated as CD103+CD11b− (CD103+ mDC), CD103−CD11b+ (CD11b+ mDC) and CD103−CD11bint/− (Langerhans cells). g, Cells from the brain, spleen, peritoneum, kidney and liver of wild-type and Mycl1+/gfp mice were stained for analysis. Shown are two-colour histograms of F4/80 and GFP expression for microglia (CD45intCD11b+), splenic red pulp macrophages (F4/80+autofluorescenthigh, RPM), peritoneum macrophages (F4/80+CD11b+), liver and kidney macrophages (F4/80+CD11bint) and liver DCs (CD11c+MHC-II+). Live haematopoietic cells were pre-gated in all non-lymphoid tissues as CD45+/int7-AAD−. Numbers represent percentage of cells in the indicated gate. Data are representative of 2–3 independent experiments (n = 4 mice). *P < 0.01; NS, P > 0.05.
Extended Data Figure 4 Mycl1 expression is restricted to DCs.
a, b, Mesenteric lymph nodes from Mycl1+/gfp (a) and Zbtb46+/gfp (b) mice were analysed for expression of B220 (blue), MAdCAM-1 (red) and GFP (green). Scale bars, 100 μm.
Extended Data Figure 5 IRF8 regulates the expression of Mycl1 in multiple DC lineages.
a, Left, bone marrow cells and splenocytes from Mycl1+/+Irf8+/+, Mycl1+/gfp Irf8+/+, Mycl1+/+Irf8R294C and Mycl1+/gfp Irf8R294C mice were stained for analysis. Shown are two-colour histograms of GFP and CD11c expression. Numbers represent the percentage of cells in the indicated gates. Data are representative of 3 independent experiments. Right, Ly6C+CD11b+ bone marrow monocytes were isolated by cell sorting from Mycl1+/+Irf8+/+(black line), Mycl1+/+Irf8R294C(grey line), Mycl1+/gfp Irf8R294C(light green line) and Mycl1+/gfp Irf8+/+mice (dark green line) and differentiated for 4 days either with GM-CSF (left panel) or with GM-CSF and IL-4 (right panel). Shown are single-colour histograms of GFP expression for pre-gated live CD11c+ cells from the indicated genotypes. b, Splenocytes from wild-type (top panels) and Irf8R294C (bottom panels) mice on the C57BL/6 genetic background were stained for analysis. Shown are two-colour histograms of CD11c and SiglecH expression (left panels), and CD11b and CD205 expression (right panels), for cells pre-gated as either B220+CD11b− or CD11c+MHC-II+B220−. pDCs were gated as CD11c+SiglecH+B220+CD11b−. Numbers represent the percentage of cells in the indicated gates. Data are representative of 2 independent experiments. c, Ly6C+ bone marrow monocytes were isolated by cell sorting from Mycl1+/+Irf8+/+, Mycl1+/+Irf8R294C, Mycl1+/gfp Irf8R294C and Mycl1+/gfp Irf8+/+ mice. Monocytes were cultured either with GM-CSF or with GM-CSF and IL-4 for 4 days and then stained for analysis. Shown are two-colour histograms of MHC-II and CD11c expression for differentiated monocytes. Numbers represent the percentage of cells in the quadrant gate. Data are representative of 2 independent experiments. d, Phoenix-E cells were transfected with murine stem cell virus retroviral plasmids expressing ERT2, murine Myc (c-Myc), murine Mycl1 (L-Myc) and a fusion between Mycl1 and the mutant human oestrogen receptor (L-Myc–ERT2). Whole-cell extracts were prepared 2 days after transfection and western blot analysis was performed. Shown are blots probed for L-Myc (left; anti-L-Myc) and c-Myc (right; anti-c-Myc) for the indicated transfections. Blots were stripped and re-probed for GFP (bottom, anti-GFP). e, IRF8 DNA binding was assayed by ChIP-seq. Shown is a histogram of normalized reads per bp per peak within a 4-kb window centred on transcriptional start sites (TSS). f, Uniquely mapped IRF8 ChIP-seq reads were evaluated for peak discovery by model-based analysis of ChIP-seq (MACS) as described in Methods and peaks were annotated by the Homer software package using default annotation settings. Shown is the distribution of peak annotations within introns, intergenic regions, promoters, transcription termination sites (TTS), 3′-untranslated regions (3′ UTR), exons and 5′-untranslated regions (5′ UTR). Numbers represent the percentage of annotations in each category. g, Peaks identified in f were evaluated for de novo motif discovery using the Homer software package as described in Methods. Analysis was performed 3 separate times to identify the most significant motifs. Shown are the top 3 DNA motifs conserved in IRF8-bound loci along with the closest known motif and associated P value (cumulative binomial distribution).
Extended Data Figure 6 Mycl1 regulates the homeostasis of CD8α+ DCs and peripheral tissue CD103+ DCs.
a, Splenic DC subsets as gated in Extended Data Fig. 1e were quantified from wild-type (black dots) and Mycl1gfp/gfp (green dots) mice on the 129S6/SvEv genetic background. Shown are graphs of total cell number per spleen for the indicated DC subsets (right panels) and of each DC subset as a percent of all splenocytes (left panels). Data are from 3 independent experiments (error bars, ± s.d.; n = 10 biological replicates, Student’s t-test). b–d, Cells from the liver (b), lung (c) and small intestine (d) of wild-type and Mycl1gfp/gfp mice on the C57BL/6 genetic background were stained for analysis. Shown are two-colour histograms of 7-AAD and CD45 expression, MHC-II and CD11c expression, and CD103 and CD11b expression. Haematopoietic cells were gated as 7-AAD−CD45+ (live CD45+) and DCs were gated as MHC-II+CD11c+. e, Cells from the lung, liver and small intestine of mixed bone marrow chimaeras described in Fig. 3a, c were analysed for relative donor contribution. Shown is the contribution of CD45.2+ wild-type bone marrow or CD45.2+ Mycl1gfp/gfp bone marrow to CD103−CD11b+ DCs in the indicated peripheral tissues as a ratio of peripheral blood neutrophils from the same animal. Data are representative of 3 independent experiments (n = 5–10 mice, Mann–Whitney U-test). f, Shown is relative Mycl1 mRNA expression determined by gene expression microarrays of lung-resident CD103+CD11b− DCs and of migratory CD103+CD11b− DCs from mediastinal lymph nodes (MLN) (error bars, s.d.; n = 3 biological replicate arrays, Student’s t-test). g, Differences in microarray-based gene expression data between lung-resident CD103+CD11b− DCs (lung, red) and migratory CD103+CD11b− DCs (lymph node, blue) were analysed for enrichment of a set of cell cycle genes (KEGG_Cell_Cycle) by Gene Set Enrichment Analysis (GSEA). Shown is a GSEA plot of running enrichment score (top), gene set member ranks (middle) and ranked list metric values (bottom) (signal-to-noise metric, n = 3 biological replicate arrays per group). **P < 0.01, ***P < 0.001; NS, P > 0.05.
Extended Data Figure 7 GM-CSF treatment and L-Myc deficiency in bone marrow pDCs, splenic CD8α+ DCs and CD8α− DCs.
a, Shown is a Venn diagram of probe sets changed at least twofold in expression between the indicated wild-type and L-Myc-deficient DC populations on gene expression microarray analysis. Indicated in each zone of the diagram is the number of probe sets. b, Shown are gene symbols and Gene Ontology (GO) biological process annotations for selected growth-related genes obtained from the analysis in a. c, CD8α+ DCs (CD11c+MHC-II+CD24+SIRP-α−B220−) and CD8α− DCs (CD11c+MHC-II+SIRP-α+CD24−B220−) were purified by cell sorting from the spleen of wild-type mice and cultured with media alone (untreated) or with GM-CSF as indicated. After 24 h, cells were analysed for viability. Shown are two-colour histograms of 7-AAD and Annexin V expression. Numbers indicate the percentage of cells in each quadrant gate. Also shown are single-colour histograms of forward scatter (FSC-A) for live cells (Annexin V−7-AAD−) to determine relative size for the indicated treatments. Data are representative of 3 independent experiments. d, Shown are Wright-Giemsa stains of cytospins prepared from CD8α+ DCs described in c. Scale bars, represent 20 μm. e, Gene expression microarray analysis was performed using CD8α+ DCs described in c. Shown is an M-plot of log2-transformed normalized expression values for probe sets either increased (red) or decreased (blue) at least twofold in expression on treatment with GM-CSF, omitting probe sets lacking gene annotations. f, Functional annotations for 500 probe sets most induced by GM-CSF treatment (e, red) were clustered using the highest classification stringency by DAVID Bioinformatics Resources. Shown are the associated GO term, enrichment score and number of contributing genes for the top 12 enriched clusters.
Extended Data Figure 8 Mycl1 is necessary for normal T-cell priming by DCs during infection.
a, Wild-type mice (top panels) and Mycl1gfp/gfp mice (bottom panels) were infected intravenously with 3 × 103 c.f.u. LM-OVA. Splenocytes were collected 7 days after infection, re-stimulated with LLO190–201 peptide in vitro for 5 h, and stained for analysis. Splenocytes from uninfected mice (control, left panel) served as a staining control. Shown are two-colour histograms of TNF-α and IFN-γ expression for cells pre-gated as Thy1.2+CD4+. Numbers represent the percent of cells in the indicated gate. Data are from 2 independent experiments. b, Shown is the total number of IFN-γ+ CD4+ T cells per spleen from the experiment described in a. Data are from 2 independent experiments (error bars, ± s.d.; n = 12 biological replicates, Student’s t-test). c, Wild-type mice (top panels) or Mycl1gfp/gfp mice (bottom panels) were infected intravenously with 5 × 106 plaque-forming units of VSV-OVA. Splenocytes were collected 7 days after infection, re-stimulated with SIINFEKL peptide in vitro for 5 h, and stained for analysis. Splenocytes from uninfected mice (control, left panel) served as a staining control. Shown are two-colour histograms of TNF-α and IFN-γ expression for cells pre-gated gated as Thy1.2+CD8α+. Numbers represent the percentage of cells in the indicated gate. Data are representative of 2 independent experiments. d, Shown is the total number of IFN-γ+ CD8+ T cells per spleen from the experiment described in c. Data are from 2 independent experiments (error bars, ± s.d.; n = 11 biological replicates, Student’s t-test). e, Wild-type mice (black dots) and Mycl1gfp/gfp mice (green dots) were infected as described in (Fig. 4f) and CD8α− DCs (CD11c+MHC-II+SIRP-α+CD24−B220−) were purified by cell sorting. Shown is the amount of viable intracellular bacteria determined for the indicated time points. Data are from 2 independent experiments (error bar, ± s.d.; n = 4–6 biological replicates). f, g, CD8α+ DCs purified from infected mice described in (Fig. 4f) were cultured for 12 h in media containing either gentamicin (f) or penicillin/streptomycin (g). CD8α+ DCs purified from uninfected mice were used as controls (uninfected, left panels). Cells were assessed for viability as in Fig. 3f. Shown are two-colour histograms of 7-AAD and Annexin V for the indicated conditions. Data are representative of 2 independent experiments. h, Mixed bone marrow chimaeras described in Fig. 3a, c were infected intravenously with 5 × 107 c.f.u. LM-EGD and splenocytes were analysed for relative donor chimaerism after 24 h. Uninfected chimaeras served as controls. Shown is the contribution of CD45.2+ wild-type bone marrow or CD45.2+ Mycl1gfp/gfp bone marrow to splenic CD8α+ DCs as a ratio of peripheral blood neutrophils from the same animal (n = 5, Mann–Whitney U-test). **P < 0.01, ***P < 0.001; NS, P > 0.05; ND, not detected.
Extended Data Figure 9 Mycl1 expression in CD8α+ DCs is necessary for normal T-cell priming.
a, Wild-type mice (black box) and BDCA2-DTR transgenic mice (open box) were treated with 125 ng diphtheria toxin (DT) per mouse 1 day before infection with 3 × 103 c.f.u. LM-OVA. Diphtheria toxin treatment was continued on days 1, 3 and 5 after infection, and splenocytes collected on day 7 were stained for analysis. Shown is the quantification of pDCs as a percentage of all splenocytes to confirm efficiency of deletion (error bars, ± s.d.; n = 4 biological replicates per group, Student’s t-test). b, Splenocytes described in a were re-stimulated with SIINFEKL peptide in vitro for 5 h. Shown is the quantification of IFN-γ+ cells as a percentage of CD8+ T cells (gated as Thy1.2+ CD8α+) (error bars, ± s.d.; n = 4 biological replicates, Student’s t-test). c, Peripheral blood from floxed (fl) Notch2fl/fl mice (black box) and Cd11c-Cre Notch2fl/fl mice (open box) was collected on day 7 after infection with 3 × 103 c.f.u. LM-OVA. After red blood cell lysis, CD8+ T cells were stained with H-2Kb-SIINFEKL tetramer. Shown is the frequency of tetramer+ cells as a percentage of CD8+ T cells (error bars, ± s.d.; n = 4 biological replicates, Student’s t-test). d, Bone marrow from CD45.1+ wild-type mice and from CD45.2+ Zbtb46dtr/dtr mice were mixed in a 50:50 ratio and injected into lethally irradiated wild-type recipient mice. Ten weeks after transplant, 400 ng diphtheria toxin was administered to each chimaeric mouse and splenocytes harvested at 48 h, 72 h and 96 h after diphtheria toxin treatment were stained for analysis. Shown are two-colour histograms of CD45.1 and CD45.2 expression for pre-gated DCs (CD11c+MHC-II+, top panels) to determine relative donor chimaerism and efficiency of deletion. e, Shown is an experimental outline for LM-OVA infection of bone marrow chimaeras following DC depletion and replenishment. f, Bone marrow chimaeras were generated using a 50:50 ratio of Zbtb46dtr/dtr bone marrow and wild-type (DTR:WT), or of Zbtb46dtr/dtr bone marrow and Mycl1gfp/gfp bone marrow (DTR:Mycl1gfp/gfp) or of Zbtb46dtr/dtr bone marrow and Batf3−/− bone marrow (DTR: Batf3−/−). Twelve weeks after lethal irradiation and transplant, bone marrow chimaeras were infected according to the time course outlined in e. Expansion of donor CD45.1+ OT-I CD8+ T cells was evaluated 7 days after infection with 3 × 102 c.f.u. LM-OVA. g, Shown is the total number of OT-I CD8+ T cells from infected DTR:WT, DTR:Mycl1gfp/gfp and DTR:Batf3−/− chimaeric mice described in e, f. Data are from 2 independent experiments (error bars, ± s.d.; n = 8 infected biological replicates, one-way ANOVA Tukey’s post-hoc test). *P < 0.05; **, P < 0.01; NS, P > 0.05.
Extended Data Figure 10 L-Myc-deficient DCs process and present soluble antigens efficiently.
a, b, CD8α+ DCs, CD8α− DCs and pDCs were isolated by cell sorting from the spleen of wild-type mice (top panels) and Mycl1gfp/gfp mice (bottom panels). OT-I CD8+ T cells and OT-II CD4+ T cells were isolated by cell sorting from the spleen of CD45.1+ OT-I transgenic and CD45.1+ OT-II transgenic mice, respectively, and T cells were then labelled with carboxyfluorescein succinimidyl ester (CFSE). DCs were pulsed with whole ovalbumin protein (Ova) for 2 h at 37 °C, then washed extensively before co-culture with OT-I CD8+ T cells (a) or OT-II CD4+ T cells (b) at a DC:T cell ratio of 1:5. Ova257–264 (OT-I CD8+ T-cell epitope) and Ova323–339 (OT-II CD4+ T-cell epitope) were used as positive controls (panels not shown). Cells were analysed 3 days later for CFSE dilution. Shown are single-colour histograms of CFSE for pre-gated live T cells. Data are representative of 2 independent experiments. c, Wild-type mice (black dots) and Mycl1gfp/gfp mice (green dots) were infected with 105 c.f.u. LM-OVA. After 24 h, CD8α+ DCs and CD8α− DCs were purified from infected spleens and co-cultured with CFSE-labelled OT-I CD45.1+ CD8+ T cells. Cells were analysed 60 h later for CFSE dilution. Shown are single-colour histograms of CFSE for live OT-I T cells gated as CD45.1+CD8α+. d, Shown is the quantitation (described in Methods) of live OT-I CD8+ T cells from c after co-culture with the indicated DCs from wild-type (black dots) and Mycl1gfp/gfp (green dots). Data are from 2 independent experiments (error bars, ± s.d.; n = 3 biological replicates, Student’s t-test). e, Histopathology (haematoxylin and eosin) of spleens (top panels) and livers (bottom panels) from wild-type (left panels), Mycl1gfp/gfp (middle panels) and Batf3−/− (right panels) mice 3 days after infection (105 L. monocytogenes i.v.). Scale bars, 200 μm. *P < 0.01; NS, P > 0.05.
Rights and permissions
About this article
Cite this article
KC, W., Satpathy, A., Rapaport, A. et al. L-Myc expression by dendritic cells is required for optimal T-cell priming. Nature 507, 243–247 (2014). https://doi.org/10.1038/nature12967
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12967
This article is cited by
-
mTOR-Myc axis drives acinar-to-dendritic cell transition and the CD4+ T cell immune response in acute pancreatitis
Cell Death & Disease (2020)
-
Regulated IRE1-dependent mRNA decay sets the threshold for dendritic cell survival
Nature Cell Biology (2017)
-
Immunogenicity is preferentially induced in sparse dendritic cell cultures
Scientific Reports (2017)
-
Glucose represses dendritic cell-induced T cell responses
Nature Communications (2017)
-
Dendritic cell metabolism
Nature Reviews Immunology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.