Abstract
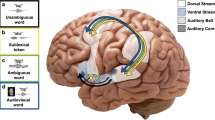
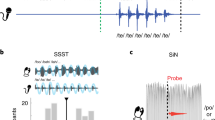
Historically, the study of speech processing has emphasized a strong link between auditory perceptual input and motor production output1,2,3,4. A kind of ‘parity’ is essential, as both perception- and production-based representations must form a unified interface to facilitate access to higher-order language processes such as syntax and semantics, believed to be computed in the dominant, typically left hemisphere5,6. Although various theories have been proposed to unite perception and production2,7, the underlying neural mechanisms are unclear. Early models of speech and language processing proposed that perceptual processing occurred in the left posterior superior temporal gyrus (Wernicke’s area) and motor production processes occurred in the left inferior frontal gyrus (Broca’s area)8,9. Sensory activity was proposed to link to production activity through connecting fibre tracts, forming the left lateralized speech sensory–motor system10. Although recent evidence indicates that speech perception occurs bilaterally11,12,13, prevailing models maintain that the speech sensory–motor system is left lateralized11,14,15,16,17,18 and facilitates the transformation from sensory-based auditory representations to motor-based production representations11,15,16. However, evidence for the lateralized computation of sensory–motor speech transformations is indirect and primarily comes from stroke patients that have speech repetition deficits (conduction aphasia) and studies using covert speech and haemodynamic functional imaging16,19. Whether the speech sensory–motor system is lateralized, like higher-order language processes, or bilateral, like speech perception, is controversial. Here we use direct neural recordings in subjects performing sensory–motor tasks involving overt speech production to show that sensory–motor transformations occur bilaterally. We demonstrate that electrodes over bilateral inferior frontal, inferior parietal, superior temporal, premotor and somatosensory cortices exhibit robust sensory–motor neural responses during both perception and production in an overt word-repetition task. Using a non-word transformation task, we show that bilateral sensory–motor responses can perform transformations between speech-perception- and speech-production-based representations. These results establish a bilateral sublexical speech sensory–motor system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Liberman, A. M., Cooper, F. S., Shankweiler, D. P. & Studdert-Kennedy, M. Perception of the Speech Code. Psychol. Rev. 74, 431–461 (1967)
Liberman, A. M. & Mattingly, I. G. The motor theory of speech perception revised. Cognition 21, 1–36 (1985)
Halle, M. & Stevens, K. N. Speech recognition: a model and a program for research. IEEE Trans. Inf. Theory 8, 155–159 (1962)
Halle, M. & Stevens, K. N. Analysis by synthesis In (eds Wathen-Dunne, W. & Woods, L. E. ) Proceedings of seminar on speech compression and processing Vol. 2 paper D7. (1959)
Berwick, R. C., Friederici, A. D., Chomsky, N. & Bolhuis, J. J. Evolution, brain, and the nature of language. Trends Cogn. Sci. 17, 89–98 (2013)
Chomsky, N. The Minimalist Program (MIT Press, 1995)
Jakobson, R. Child Language, Aphasia and Phonological Universals (Mouton, 1968)
Lichtheim, L. On aphasia. Brain 7, 433–484 (1885)
Wernicke, C. The aphasic symptom-complex: a psychological study on an anatomical basis. Arch. Neurol. 22, 280–282 (1970)
Geschwind, N. Disconnexion syndromes in animals and man. I. Brain 88, 237–294 (1965)
Hickok, G. & Poeppel, D. The cortical organization of speech processing. Nature Rev. Neurosci. 8, 393–402 (2007)
Price, C. J. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann. NY Acad. Sci. 1191, 62–88 (2010)
Obleser, J., Eisner, F. & Kotz, S. A. Bilateral speech comprehension reflects differential sensitivity to spectral and temporal features. J. Neurosci. 28, 8116–8123 (2008)
Rauschecker, J. P. & Scott, S. K. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nature Rev. Neurosci. 12, 718–724 (2009)
Hickok, G., Houde, J. & Rong, F. Sensorimotor integration in speech processing: computational basis and neural organization. Neuron 69, 407–422 (2011)
Hickok, G., Okada, K. & Serences, J. T. Area Spt in the human planum temporale supports sensory-motor integration for speech processing. J. Neurophysiol. 101, 2725–2732 (2009)
Guenther, F. H. Cortical interactions underlying the production of speech sounds. J. Commun. Disord. 39, 350–365 (2006)
Wise, R. J. S. et al. Separate neural subsystems within ‘Wernicke’s area’. Brain 124, 83–95 (2001)
Caramazza, A., Basili, A. G., Koller, J. J. & Berndt, R. S. An investigation of repetition and language processing in a case of conduction aphasia. Brain Lang. 14, 235–271 (1981)
Crone, N. E., Sinai, A. & Korzeniewska, A. Event-related dynamics of brain oscillations. Prog. Brain Res. 159, 275–295 (2006)
Markowitz, D. A., Wong, Y. T., Gray, C. M. & Pesaran, B. Optimizing the decoding of movement goals from local field potentials in macaque cortex. J. Neurosci. 31, 18412–18422 (2011)
Zhang, M. & Barash, S. Neuronal switching of sensorimotor transformations for antisaccades. Nature 408, 971–975 (2000)
Gail, A. & Andersen, R. Neural dynamics in monkey parietal reach region reflect context-specific sensorimotor transformations. J. Neurosci. 26, 9376–9384 (2006)
Chang, E. F., Niziolek, C. A., Knight, R. T., Nagarajan, S. S. & Houde, J. F. Human cortical sensorimotor network underlying feedback control of vocal pitch. Proc. Natl Acad. Sci. USA 110, 2653–2658 (2013)
Oller, D. K., Eilers, R. E. & Oiler, D. K. The role of audition in infant babbling the role of audition in infant babbling. Child Dev. 59, 441–449 (1988)
Agnew, Z. K., McGettigan, C., Banks, B. & Scott, S. K. Articulatory movements modulate auditory responses to speech. Neuroimage 73, 191–199 (2013)
Goodglass, H., Kaplan, E. & Barresi, B. Assessment of Aphasia and Related Disorders (Lippincott Williams & Wilkins, 2000)
Damasio, H. & Damasio, A. R. The anatomical basis of conduction aphasia. Brain 103, 337–350 (1980)
Benson, D. F. et al. Conduction aphasia: a clinicopathic study. Arch. Neurol. 28, 339–346 (1973)
Murphy, K. et al. Cerebral areas associated with motor control of speech in humans cerebral areas associated with motor control of speech in humans. J. Appl. Physiol. 83, 1438–1447 (1997)
Yang, A. I. et al. Localization of dense intracranial electrode arrays using magnetic resonance imaging. NeuroImage 63, 157–165 (2012)
Kovalev, D. et al. Rapid and fully automated visualization of subdural electrodes in the presurgical evaluation of epilepsy patients. AJNR Am. J. Neuroradiol. 26, 1078–1083 (2005)
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-based analysis. I: segmentation and surface reconstruction. Neuroimage 9, 179–194 (1999)
Mitra, P. P. & Pesaran, B. Analysis of dynamic brain imaging data. Biophys. J. 76, 691–708 (1999)
Maris, E., Schoffelen, J.-M. & Fries, P. Nonparametric statistical testing of coherence differences. J. Neurosci. Methods 163, 161–175 (2007)
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995)
Acknowledgements
We would like to thank A. Weiss, J. MacArthur and L. Frank for developing the data acquisition hardware and software; O. Felsovalyi, E. Londen, P. Purushothaman, L. Melloni, C. Boomhaur and A. Trongnetrpunya for technical assistance; and D. Poeppel and C. Brody for comments on the manuscript. This work was supported, in part, by R03-DC010475 from the NIDCD, a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund (B.P.), a Watson Investigator Program Award from NYSTAR (B.P.), a McKnight Scholar Award (B.P.) and a Sloan Research Fellowship (B.P.).
Author information
Authors and Affiliations
Contributions
G.B.C. designed the experiment, performed the research, analysed the data and wrote the manuscript. T.T. and O.D. performed the research and wrote the manuscript. C.C. and W.D. performed the research. B.P. designed the experiment, performed the research, analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion, Supplementary Tables 1-2, Supplementary Figures 1-11 and additional references. (PDF 2214 kb)
Rights and permissions
About this article
Cite this article
Cogan, G., Thesen, T., Carlson, C. et al. Sensory–motor transformations for speech occur bilaterally. Nature 507, 94–98 (2014). https://doi.org/10.1038/nature12935
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12935
This article is cited by
-
A neural speech decoding framework leveraging deep learning and speech synthesis
Nature Machine Intelligence (2024)
-
Decoding Single and Paired Phonemes Using 7T Functional MRI
Brain Topography (2024)
-
Cerebral activation caused by dental sounds: a functional magnetic resonance imaging study
Odontology (2024)
-
High-resolution neural recordings improve the accuracy of speech decoding
Nature Communications (2023)
-
A speech planning network for interactive language use
Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.