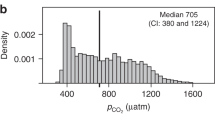
Abstract
River systems connect the terrestrial biosphere, the atmosphere and the ocean in the global carbon cycle1. A recent estimate suggests that up to 3 petagrams of carbon per year could be emitted as carbon dioxide (CO2) from global inland waters, offsetting the carbon uptake by terrestrial ecosystems2. It is generally assumed that inland waters emit carbon that has been previously fixed upstream by land plant photosynthesis, then transferred to soils, and subsequently transported downstream in run-off. But at the scale of entire drainage basins, the lateral carbon fluxes carried by small rivers upstream do not account for all of the CO2 emitted from inundated areas downstream3,4. Three-quarters of the world’s flooded land consists of temporary wetlands5, but the contribution of these productive ecosystems6 to the inland water carbon budget has been largely overlooked. Here we show that wetlands pump large amounts of atmospheric CO2 into river waters in the floodplains of the central Amazon. Flooded forests and floating vegetation export large amounts of carbon to river waters and the dissolved CO2 can be transported dozens to hundreds of kilometres downstream before being emitted. We estimate that Amazonian wetlands export half of their gross primary production to river waters as dissolved CO2 and organic carbon, compared with only a few per cent of gross primary production exported in upland (not flooded) ecosystems1,7. Moreover, we suggest that wetland carbon export is potentially large enough to account for at least the 0.21 petagrams of carbon emitted per year as CO2 from the central Amazon River and its floodplains8. Global carbon budgets should explicitly address temporary or vegetated flooded areas, because these ecosystems combine high aerial primary production with large, fast carbon export, potentially supporting a substantial fraction of CO2 evasion from inland waters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cole, J. J. et al. Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems 10, 172–185 (2007)
Aufdenkampe, A. K. et al. Rivers key to coupling biogeochemical cycles between land, oceans and atmosphere. Front. Ecol. Environ. 9, 53–60 (2011)
Davidson, E. A., Figueiredo, R. O., Markewitz, D. & Aufdenkampe, A. K. Dissolved CO2 in small catchment streams of eastern Amazonia: a minor pathway of terrestrial carbon loss. J. Geophys. Res. 115, G04005 (2010)
Butman, D. & Raymond, P. A. Significant efflux of carbon dioxide from streams and rivers in the United States. Nature Geosci. 4, 839–842 (2011)
Downing, J. A. Global limnology: up-scaling aquatic services and processes to planet Earth. Verh. Int. Verein. Limnol. 30, 1149–1166 (2009)
Whittaker, R. H. & Likens, G. E. in Primary Productivity of the Biosphere (eds Lieth, H. & Whittaker, R. H. ) 305–328 (Springer, 1975)
Schulze, E. D. et al. The European carbon balance. Part 4: integration of carbon and other trace-gas fluxes. Glob. Change Biol. 16, 1451–1469 (2010)
Richey, J. E., Melack, J. M., Aufdenkampe, A. K., Ballester, V. M. & Hess, L. L. Outgassing from Amazonian rivers and wetlands as a large tropical source of atmospheric CO2 . Nature 416, 617–620 (2002)
Battin, T. J. et al. Biophysical controls on organic carbon fluxes in fluvial networks. Nature Geosci. 1, 95–100 (2008)
Duarte, C. M. & Prairie, Y. T. Prevalence of heterotrophy and atmospheric CO2 emissions from aquatic ecosystems. Ecosystems 8, 862–870 (2005)
Kayranli, B., Scholz, M., Mustafa, A. & Hedmark, A. Carbon storage and fluxes within freshwater wetlands: a critical review. Wetlands 30, 111–124 (2010)
Hess, L. L., Melack, J. M., Novo, E. M., Barbosa, C. C. F. & Gastil, M. Dual-season mapping of wetland inundation and vegetation for the central Amazon basin. Remote Sens. Environ. 87, 404–428 (2003)
Maurice-Bourgoin, L. et al. Temporal dynamics of water and sediment exchanges between the Curuaí floodplain and the Amazon River, Brazil. J. Hydrol. 335, 140–156 (2007)
Schöngart, J., Wittmann, F. & Worbes, M. in Amazonian Floodplain Forests: Ecophysiology, Biodiversity and Sustainable Management (eds Junk, W. J. et al.) 347–388 (Springer, 2010)
Engle, D. L., Melack, J. M., Doyle, R. D. & Fisher, T. R. High rates of net primary production and turnover of floating grasses on the Amazon floodplain: implications for aquatic respiration and regional CO2 flux. Glob. Change Biol. 14, 369–381 (2008)
Hamilton, S. K., Sippel, S. J. & Melack, J. M. Oxygen depletion and carbon dioxide and methane production in waters of the Pantanal wetland of Brazil. Biogeochemistry 30, 115–141 (1995)
Polsenaere, P. et al. Thermal enhancement of gas transfer velocity of CO2 in an Amazon floodplain lake revealed by eddy covariance. Geophys. Res. Lett. 40, 1734–1740 (2013)
Junk, W. J., Bayley, P. B. & Sparks, R. E. The flood pulse concept in river–floodplain systems. in Proc. Int. Large River Symp. (ed. Dodge, D. P. ) Can. J. Fish. Aquat. Sci. Spec. Publ. 106, 110–127 (1989)
Devol, A. H. et al. Seasonal variation in chemical distributions in the Amazon (Solimões) River: a multiyear time series. Glob. Biogeochem. Cycles 9, 307–328 (1995)
Ellis, E. E. et al. Factors controlling water-column respiration in rivers of the central and southwestern Amazon Basin. Limnol. Oceanogr. 57, 527–540 (2012)
Mortillaro, J. M. et al. Particulate organic matter distribution along the Lower Amazon River: addressing aquatic ecology concepts using fatty acids. PLoS ONE 7, e46141 (2012)
Moreira-Turcq, P. et al. Seasonal variability in concentration, composition, age and fluxes of particulate organic carbon exchanged between the floodplain and Amazon River. Glob. Biogeochem. Cycles 27, 119–130 (2013)
Quay, P. D. et al. Carbon cycling in the Amazon River: implications from the 13C compositions of particles and solutes. Limnol. Oceanogr. 37, 857–871 (1992)
Ward, N. D. et al. Degradation of terrestrially derived macromolecules in the Amazon River. Nature Geosci. 6, 530–533 (2013)
Mayorga, E. et al. Young organic matter as a source of carbon dioxide outgassing from Amazonian rivers. Nature 436, 538–541 (2005)
Worbes, M. in The Central Amazon Floodplain: Ecology of a Pulsing System (ed. Junk, W. J. ) 223–265 (Springer, 1997)
Lloyd, J. et al. An airborne regional carbon balance of Central Amazonia. Biogeosciences 4, 759–768 (2007)
Aselmann, I. & Crutzen, P. J. Global distribution of natural freshwater wetlands and rice paddies, their net primary productivity, seasonality and possible methane emissions. J. Atmos. Chem. 8, 307–358 (1989)
Abril, G., Richard, S. & Guérin, F. In situ measurements of dissolved gases (CO2 and CH4) in a wide range of concentrations in a tropical reservoir using an equilibrator. Sci. Total Environ. 354, 246–251 (2006)
Sioli, H. Hydrochemistry and geology in the Brazilian Amazon region. Amazoniana 3, 267–277 (1968)
Mertes, L. A. K., Dunne, T. & Martinelli, L. A. Channel–floodplain geomorphology along the Solimões-Amazon River, Brazil. Geol. Soc. Am. Bull. 108, 1089–1107 (1996)
Trigg, M. A., Bates, P. D., Wilson, M. D., Schumann, G. & Baugh, C. Floodplain channel morphology and networks of the middle Amazon River. Wat. Resour. Res. 48, W10504 (2012)
Alsdorf, D., Han, S.-C., Bates, P. & Melack, J. Seasonal water storage on the Amazon floodplain measured from satellites. Remote Sens. Environ. 114, 2448–2456 (2010)
Bonnet, M. P. et al. Floodplain hydrology in an Amazon floodplain lake (Lago Grande de Curuai). J. Hydrol. 349, 18–30 (2008)
Rosenqvist, A., Forsberg, B. R., Pimentel, T., Rauste, Y. A. & Richey, J. E. The use of spaceborne radar to model inundation patterns and trace gas emissions in the central Amazon floodplain. Int. J. Remote Sens. 23, 1303–1328 (2002)
Martinez, J. M. & Le Toan, T. Mapping of flood dynamics and spatial distribution of vegetation in the Amazon floodplain using multitemporal SAR data. Remote Sens. Environ. 108, 209–223 (2007)
Quegan, S., Le Toan, T., Yu, J. J., Ribbes, F. & Floury, N. Multitemporal ERS SAR analysis applied to forest monitoring. IEEE Trans. Geosci. Rem. Sens. 38, 741–753 (2000)
Lee, J. S. A simple speckle smoothing algorithm for synthetic aperture radar images. IEEE Trans. Syst. Man Cybern. 13, 85–89 (1983)
Frankignoulle, M., Borges, A. & Biondo, R. A new design of equilibrator to monitor carbon dioxide in highly dynamic and turbid environments. Water Res. 35, 1344–1347 (2001)
Santos, I. R., Maher, D. T. & Eyre, B. D. Coupling automated radon and carbon dioxide measurements in coastal waters. Environ. Sci. Technol. 46, 7685–7691 (2012)
Beutler, M. et al. A fluorometric method for the differentiation of algal populations in vivo and in situ. Photosynth. Res. 72, 39–53 (2002)
MacIntyre, H. L., Lawrenz, E. & Richardson, T. L. in Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications (eds Suggett, D. J. et al.) 129–169 (Developments in Applied Phycology 4, Springer, 2010)
Lorenzen, C. J. Determination of chlorophyll and pheopigments: spectrophotometric equations. Limnol. Oceanogr. 12, 343–346 (1967)
Weiss, R. F. Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Mar. Chem. 2, 203–215 (1974)
Jähne, B. et al. On parameters influencing air-water exchange. J. Geophys. Res. 92, 1937–1949 (1987)
Wanninkhof, R. Relationship between gas exchange and wind speed over the ocean. J. Geophys. Res. 97, 7373–7382 (1992)
Guérin, F. et al. Gas transfer velocities of CO2 and CH4 in a tropical reservoir and its river downstream. J. Mar. Syst. 66, 161–172 (2007)
Cole, J. J. & Caraco, N. F. Atmospheric exchange of carbon dioxide in a low-wind oligotrophic lake measured by the addition of SF6 . Limnol. Oceanogr. 43, 647–656 (1998)
Zappa, C. J. et al. Environmental turbulent mixing controls on air-water gas exchange in marine and aquatic systems. Geophys. Res. Lett. 34, http://dx.doi.org/10.1029/2006GL028790 (2007)
Abril, G., Commarieu, M. V., Sottolichio, A., Bretel, P. & Guérin, F. Turbidity limits gas exchange in a large macrotidal estuary. Estuar. Coast. Shelf Sci. 83, 342–348 (2009)
MacIntyre, S. et al. Buoyancy flux, turbulence, and the gas transfer coefficient in a stratified lake. Geophys. Res. Lett. 37, L24604 (2010)
Rudorff, C. M., Melack, J. M., MacIntyre, S., Barbosa, C. C. F. & Novo, E. M. L. M. Seasonal and spatial variability of CO2 emission from a large floodplain lake in the lower Amazon. J. Geophys. Res. 116, G04007 (2011)
Salter, M. E. et al. Impact of an artificial surfactant release on air-sea gas fluxes during deep ocean gas exchange experiment II. J. Geophys. Res. 116, C11016 (2011)
Parolin, P. et al. Central Amazon floodplain forests: tree survival in a pulsing system. Bot. Rev. 70, 357–380 (2004)
Richey, J. E., Krusche, A. V., Johnson, M. S., da Cunha, H. B. & Ballester, M. V. in Amazonia and Global Change (eds Keller, M. et al.) 489–504 (Geophys. Monogr. Ser. 186, AGU, 2009)
Devol, A. H., Quay, P. D., Richey, J. E. & Martinelli, L. A. The role of gas exchange in the inorganic carbon, oxygen and 222Rn budgets of the Amazon River. Limnol. Oceanogr. 32, 235–248 (1987)
Alin, S. R. et al. Physical controls on carbon dioxide transfer velocity and flux in low-gradient river systems and implications for regional carbon budgets. J. Geophys. Res. 116, G01009 (2011)
Junk, W. J. & Piedade, M. T. F. Biomass and primary production of herbaceous plant communities in the Amazon floodplain. Hydrobiology 263, 155–162 (1993)
Malhi, Y. & Grace, J. Tropical forests and atmospheric carbon dioxide. Trees 15, 332–337 (2000)
Horna, V., Zimmermann, R., Müller, E. & Parolin, P. in Amazonian Floodplain Forests: Ecophysiology, Biodiversity and Sustainable Management (eds Junk, W. J. et al.) 223–241 (Springer, 2010)
Worbes, M. in The Central Amazon Floodplain: Ecology of a Pulsing System (ed Junk, W. J. ) 223–265 (Springer, 1997)
Saatchi, S. S., Houghton, R. A., Dos Santos Avala, R. C., Soares, J. V. & Yu, Y. Distribution of aboveground live biomass in the Amazon basin. Glob. Change Biol. 13, 816–837 (2007)
Meyer, U., Junk, W. J. & Linck, C. in Amazonian Floodplain Forests: Ecophysiology, Biodiversity and Sustainable Management (eds Junk, W. J. et al.) 163–178 (Springer, 2010)
Parolin, P., Wittmann, F. & Schöngart, J. in Amazonian Floodplain Forests: Ecophysiology, Biodiversity and Sustainable Management (eds Junk, W. J. et al.) 105–126 (Springer, 2010)
Piedade, M. T. F., Ferreira, C. S., de Oliveira Wittmann, A., Buckeridge, M. & Parolin, P. in Amazonian Floodplain Forests: Ecophysiology, Biodiversity and Sustainable Management (eds Junk, W. J. et al.) 127–139 (Springer, 2010)
Junk, W. J., Piedade, M. T. F., Parolin, P., Wittmann, F. & Schöngart, J. in Amazonian Floodplain Forests: Ecophysiology, Biodiversity and Sustainable Management (eds Junk, W. J. et al.) 511–540 (Springer, 2010)
Morison, J. I. L. et al. Very high productivity of the C4 aquatic grass Echinocloa polystachya in the Amazon floodplain confirmed by net ecosystem CO2 flux measurements. Oecologia 125, 400–411 (2000)
Costa, M. Estimate of net primary productivity of aquatic vegetation of the Amazon floodplain using Radarsat and JERS-1. Int. J. Remote Sens. 26, 4527–4536 (2005)
Melack, J. M. et al. in Amazonia and Global Change (eds Keller, M. et al.) 525–542 (Geophys. Monogr. Ser. 186, AGU, 2009)
Moreira-Turcq, P. et al. Carbon sedimentation at Lago Grande de Curuai, a floodplain lake in the low Amazon region: insights into sedimentation rates. Palaeogeogr. Palaeoclim. Palaeoecol. 214, 27–40 (2004)
Acknowledgements
This research is a contribution to the CARBAMA project, funded by the French National Agency for Research (grant number 08-BLAN-0221), the French INSU national programme EC2CO, and the National Council of Research and Development (CNPq), Brazil (Universal Program number 477655/2010-6). It was conducted under the auspices of the Environmental Research Observatory Hydrology and Geochemistry of the Amazon Basin (HYBAM), supported by the INSU and the IRD (Institute for Research and Development, France). F.R. was supported by CNPq and a Brazilian ‘Excellent Researcher’ fellowship. We thank all the participants of the CARBAMA cruises.
Author information
Authors and Affiliations
Contributions
G.A., J.-M.M., P.M.-T., L.F.A., T.M. and M.F.B. conceived and designed the study. G.A. coordinated project and fieldwork. G.A., J.D., M.F.L.d.S. and N.S. performed the measurements. J.-M.M. and E.L.S. analysed the remote sensing data. L.F.A. measured Chl a and fluorescence. L.V. and F.R. measured respiration. All authors contributed to the interpretation of the data. G.A. wrote the manuscript, J.-M.M., L.F.A. and F.R. contributed to manuscript writing and P.M.-T., L.V., T.M., J.-H.K., M.C.B., N.S. and M.F.B. commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Instrumental set-up for continuous measurement of and ancillary parameters while underway in the Amazon River and floodplain lakes.
Side-view diagrams of both boats are illustrated with photos of the equipment. See detailed description in the Methods.
Extended Data Figure 2 Continuous record of water in the Amazon River, tributaries, and floodplain lakes during the high water (June 2009).
a, Track of the ship in the main stem (brown), floodplain lakes (green), and major tributaries (blue). Land occupation derived from SAR data is shown as flooded forest (light grey), temporary open waters (dark grey) and permanent open waters (black). b, Conductivity values show that water in the floodplain lakes primarily originates from the flooding of the Solimões and Amazon rivers with modest contribution from local drainage. c, The distribution of water  at maximum flooding shows the predominance of supersaturation with a net decrease downstream, in parallel with the extent of vegetation in the floodplains (percentage of total floodplain area is given in parentheses for each lake).
at maximum flooding shows the predominance of supersaturation with a net decrease downstream, in parallel with the extent of vegetation in the floodplains (percentage of total floodplain area is given in parentheses for each lake).
Extended Data Figure 3 Continuous record of water in the Amazon River, its tributaries and floodplain lakes during the low water of October 2009.
a, Track of the ship in the main stem (brown), floodplain lakes (green), and major tributaries (blue). Land occupation derived from SAR data is shown as flooded forest (light grey), temporary open waters (dark grey) and permanent open waters (black). b, Conductivity values show that water in the floodplain lakes primarily originates from the flooding of the Solimões and Amazon rivers with modest contribution from local drainage. c, The distribution of water  shows large contrasts between channel and floodplains, with a significant decrease downstream (as during the high water), in parallel with the extent of vegetation in the floodplains (percentage of total floodplain area is given in parentheses for each lake). Undersaturation in
shows large contrasts between channel and floodplains, with a significant decrease downstream (as during the high water), in parallel with the extent of vegetation in the floodplains (percentage of total floodplain area is given in parentheses for each lake). Undersaturation in  occurs at low water in dense phytoplankton blooms in almost isolated lakes.
occurs at low water in dense phytoplankton blooms in almost isolated lakes.
Extended Data Figure 4 Conceptual diagram for carbon dioxide outgassing fuelled by Amazonian wetlands.
In flooded forests, aerial gross primary production absorbs CO2 from the atmosphere and sequesters part of this carbon in wood. Most of the sequestration in wood occurs during the terrestrial phase and is supposed to be balanced by natural tree mortality associated with channel migration. Leaves and wood also respire CO2 back to the atmosphere. Litter falls from flooded trees primarily during flooding and constitutes a significant organic carbon input to the water. Floating plants in the Amazon grow above the water level, where they perform aerial photosynthesis, and as the water level progressively rises, their biomass is recycled and decomposes underwater. Because no significant burial of macrophyte material is observed in sediment, it is assumed that all their annual net primary production (NPP) is transferred to water as organic carbon (litter fall). Below water, the respiration of roots of flooded trees and floating macrophytes releases CO2 to the water. With the establishment of anoxic conditions in forest soils, tree metabolism deviates to an anaerobic pathway that generates fermentation products, which are exuded from the roots into the surrounding water. Carbon flux between the Amazonian wetlands and rivers thus occurs through two distinct pathways. CO2 export from the wetlands is derived from root and sediment respiration within the wetlands, whereas organic carbon export from the wetlands is derived from litter fall and from fermentative products released by roots. Quantitative information is missing for the latter exudation flux. In rivers and floodplains, water movement is fast enough relative to gas exchange to generate a lateral CO2 flux with the water mass, and this flux should be taken into account in the interpretation of the spatial and temporal patterns of CO2 outgassing. In water and sediments of the entire aquatic system, microbial heterotrophic respiration continuously converts organic carbon to CO2. In open lakes, phytoplankton uses CO2 dissolved in water (that is, primarily derived from the surrounding wetland vegetation) and infrequently uses atmospheric CO2 because the lakes were rarely net CO2 sinks on a daily basis. The phytoplankton biomass produced in open lakes constitutes an additional source of biodegradable organic carbon. Both C3 and C4 plants are well represented in the wetland. Isotopic and molecular tracers may distinguish woody from non-woody material. However, it is difficult to differentiate woody material from the flooded forest and woody material from the non-inundated forest, particularly as many species are common to both. More detailed discussion and references can be found in the Supplementary Information.
Extended Data Figure 5 Modelling how far dissolved CO2 is transported before being outgassed.
a, We assessed the potential for lateral CO2 transport in rivers and floodplains using a simple one-dimensional model that simultaneously calculates the CO2 lost by outgassing and the CO2 that remains dissolved in water and is transported downstream by the currents. The model starts from a point source in the wetland (set here at 12,000 p.p.m.v., which is a typical value observed in the vicinity of a flooded forest; Fig. 2b). The iteration time was 1 min. In the model, F(CO2) is calculated from  , using representative values of k600. The quantity of CO2 lost to the atmosphere during one iteration is subtracted from the initial CO2 quantity present in a column of water of a determined depth H. Note that this procedure is adequate only for acidic, non-buffered waters, such as those in the Amazon. b, c, When integrated (Supplementary Information), the equation gives a one-phase exponential decay function of
, using representative values of k600. The quantity of CO2 lost to the atmosphere during one iteration is subtracted from the initial CO2 quantity present in a column of water of a determined depth H. Note that this procedure is adequate only for acidic, non-buffered waters, such as those in the Amazon. b, c, When integrated (Supplementary Information), the equation gives a one-phase exponential decay function of  versus the distance x, the water current velocity w, the normalized gas transfer velocity k600, and the water depth H. The curves give the potential extent of
versus the distance x, the water current velocity w, the normalized gas transfer velocity k600, and the water depth H. The curves give the potential extent of  saturation that can be maintained without the necessity of aquatic respiration (Fig. 2b). D½ is the half-evasion distance, which is the theoretical distance the water mass travels before outgassing half of its initial excess CO2. T½ is the associated half-evasion time. d, Typical half-evasion distances of wetland CO2 in river–floodplain systems vary from less than 1 km in a shallow, stagnant, wind- and heat-protected lake to more than 300 km in a deep and fast-flowing river. This illustrates, on the one hand, how far wetland CO2 can be exported downstream, and on the other hand, the large heterogeneity of the transport and outgassing processes in the river–floodplain complex.
saturation that can be maintained without the necessity of aquatic respiration (Fig. 2b). D½ is the half-evasion distance, which is the theoretical distance the water mass travels before outgassing half of its initial excess CO2. T½ is the associated half-evasion time. d, Typical half-evasion distances of wetland CO2 in river–floodplain systems vary from less than 1 km in a shallow, stagnant, wind- and heat-protected lake to more than 300 km in a deep and fast-flowing river. This illustrates, on the one hand, how far wetland CO2 can be exported downstream, and on the other hand, the large heterogeneity of the transport and outgassing processes in the river–floodplain complex.
Supplementary information
Supplementary Information
This file contains Supplementary Text 1-4 and additional references. (PDF 409 kb)
Rights and permissions
About this article
Cite this article
Abril, G., Martinez, JM., Artigas, L. et al. Amazon River carbon dioxide outgassing fuelled by wetlands. Nature 505, 395–398 (2014). https://doi.org/10.1038/nature12797
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12797
This article is cited by
-
Characteristics of soil organic carbon fractions in four vegetation communities of an inland salt marsh
Carbon Balance and Management (2024)
-
Revealing the hidden carbon in forested wetland soils
Nature Communications (2024)
-
Dissolved greenhouse gas (CO2, CH4, and N2O) emissions from highland lakes of the Andes cordillera in Northern Ecuador
Aquatic Sciences (2024)
-
Eco-morphodynamic carbon pumping by the largest rivers in the Neotropics
Scientific Reports (2023)
-
Andean headwater and piedmont streams are hot spots of carbon dioxide and methane emissions in the Amazon basin
Communications Earth & Environment (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.