Abstract
Circadian oscillation of body temperature is a basic, evolutionarily conserved feature of mammalian biology1. In addition, homeostatic pathways allow organisms to protect their core temperatures in response to cold exposure2. However, the mechanism responsible for coordinating daily body temperature rhythm and adaptability to environmental challenges is unknown. Here we show that the nuclear receptor Rev-erbα (also known as Nr1d1), a powerful transcriptional repressor, links circadian and thermogenic networks through the regulation of brown adipose tissue (BAT) function. Mice exposed to cold fare considerably better at 05:00 (Zeitgeber time 22) when Rev-erbα is barely expressed than at 17:00 (Zeitgeber time 10) when Rev-erbα is abundant. Deletion of Rev-erbα markedly improves cold tolerance at 17:00, indicating that overcoming Rev-erbα-dependent repression is a fundamental feature of the thermogenic response to cold. Physiological induction of uncoupling protein 1 (Ucp1) by cold temperatures is preceded by rapid downregulation of Rev-erbα in BAT. Rev-erbα represses Ucp1 in a brown-adipose-cell-autonomous manner and BAT Ucp1 levels are high in Rev-erbα-null mice, even at thermoneutrality. Genetic loss of Rev-erbα also abolishes normal rhythms of body temperature and BAT activity. Thus, Rev-erbα acts as a thermogenic focal point required for establishing and maintaining body temperature rhythm in a manner that is adaptable to environmental demands.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bass, J. Circadian topology of metabolism. Nature 491, 348–356 (2012)
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004)
Takahashi, J. S., Hong, H.-K., Ko, C. H. & McDearmon, E. L. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nature Rev. Genet. 9, 764–775 (2008)
Sahar, S. & Sassone-Corsi, P. Metabolism and cancer: the circadian clock connection. Nature Rev. Cancer 9, 886–896 (2009)
Asher, G. & Schibler, U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 13, 125–137 (2011)
Bass, J. & Takahashi, J. S. Circadian integration of metabolism and energetics. Science 330, 1349–1354 (2010)
Feng, D. & Lazar, M. A. Clocks, metabolism, and the epigenome. Mol. Cell 47, 158–167 (2012)
Buhr, E. D., Yoo, S.-H. & Takahashi, J. S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330, 379–385 (2010)
Feng, D. et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science 331, 1315–1319 (2011)
Woldt, E. et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nature Med. (2013)
Bugge, A. et al. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 26, 657–667 (2012)
Cho, H. et al. Regulation of circadian behaviour and metabolism by Rev-erb-α and Rev-erb-β. Nature 485, 123–127 (2012)
Delezie, J. et al. The nuclear receptor Rev-erbα is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 26, 3321–3335 (2012)
Le Martelot, G. et al. Rev-erbα participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 7, e1000181 (2009)
Solt, L. A. et al. Regulation of circadian behaviour and metabolism by synthetic Rev-erb agonists. Nature 485, 62–68 (2012)
Cannon, B. & Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 214, 242–253 (2011)
Preitner, N. et al. The orphan nuclear receptor Rev-erbα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002)
Lim, S. et al. Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nature Protocols 7, 606–615 (2012)
Golozoubova, V. et al. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 15, 2048–2050 (2001)
Talan, M. I., Tatelman, H. M. & Engel, B. T. Cold tolerance and metabolic heat production in male C57BL/6J mice at different times of day. Physiol. Behav. 50, 613–616 (1991)
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998)
Pearen, M. A. et al. The orphan nuclear receptor, NOR-1, is a target of β-adrenergic signaling in skeletal muscle. Endocrinology 147, 5217–5227 (2006)
Cypess, A. M. et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc. Natl Acad. Sci. USA 109, 10001–10005 (2012)
Vosselman, M. J. et al. Systemic β-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes 61, 3106–3113 (2012)
Nedergaard, J. & Cannon, B. UCP1 mRNA does not produce heat. Biochim. Biophys. Acta 1831, 943–949 (2013)
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009)
Van Marken Lichtenbelt, W. D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009)
Virtanen, K. A. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 (2009)
Van der Veen, D. R., Shao, J., Chapman, S., Leevy, W. M. & Duffield, G. E. A diurnal rhythm in glucose uptake in brown adipose tissue revealed by in vivo PET-FDG imaging. Obesity 20, 1527–1529 (2012)
Saito, S., Saito, C. T. & Shingai, R. Adaptive evolution of the uncoupling protein 1 gene contributed to the acquisition of novel nonshivering thermogenesis in ancestral eutherian mammals. Gene 408, 37–44 (2008)
Acknowledgements
We thank the Functional Genomics Core (J. Schug) and the Mouse Phenotyping, Physiology, and Metabolism Core (R. Ahima and R. Dhir) of the Penn Diabetes Research Center (NIH P30 DK19525). We also thank the Small Animal Imaging Facility of the Perelman School of Medicine at the University of Pennsylvania (E. Blankemeyer). This work was supported by NIH grants R01 DK45586 (M.A.L.) and F-32 DK095563 (Z.G.-H.) and the JPB Foundation. A.B. was funded by the Novo Nordisk STAR postdoctoral program.
Author information
Authors and Affiliations
Contributions
D.F., M.J.E., L.J.E., E.R.B., A.B. and C.F. performed key experiments/data analysis and read the manuscript. P.S. provided advice and read the manuscript. E.L. and T.S.K. designed, performed and analysed EMG studies and read the manuscript. C.H. and D.A.P. designed, performed and analysed 18FDG scans and read the manuscript. Z.G.H. performed many of the experiments, and Z.G.H. and M.A.L. conceived the project, designed experiments, analysed all results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 The BAT core clock is largely unaffected by Rev-erbα deletion.
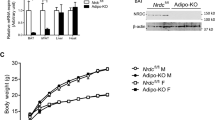
a, Rev-erbα protein levels in BAT of wild-type and Rev-erbα knockout mice (n = 2; each lane of the western blot represents pooled biological duplicates). b, BAT mRNA for indicated genes from wild-type and Rev-erbα knockout mice collected at the indicated times over a 24-h time course (n = 3).
Extended Data Figure 2 Rev-erbα controls cold and noradrenaline-induced oxidative metabolism independently of skeletal muscle metabolism.
a, Food intake from cold-challenged Rev-erbα knockout mice and control littermates in Fig. 1f. b, r.m.s. derivation of EMG measurement from Fig. 1g. c, Oxygen consumption rates of Rev-erbα KO mice and control littermates following noradrenaline administration (1 mg kg−1 s.c.) (n = 6). d, e, r.m.s. derivation of EMG measurements performed on wild-type and Rev-erbα knockout mice following noradrenaline administration (1 mg kg−1 s.c.) (n = 4). ***P < 0.001 as determined by Student’s t-test. Data are expressed as mean ± s.d.
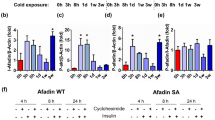
Extended Data Figure 3 Rev-erbα, but not Rev-erbβ, is decreased in a cold-dependent manner.
a–c, Rev-erbβ (a), Bmal1 (b) and Pgc1a (c) mRNA levels in BAT during a cold-exposure time course (n = 3 for mRNA). d, BAT gene expression following moderate (20 °C) or acute (4 °C) cold challenges (n = 3). e, BAT protein levels after 3 h noradrenaline administration (1 mg kg−1 i.p.) or cold exposure (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 as determined by one-way ANOVA with multiple comparisons and a Tukey’s post-test. Data are expressed as mean ± s.d.
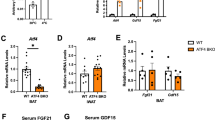
Extended Data Figure 4 Rev-erbα negatively regulates Ucp1.
a, b, BAT mRNA (a) and protein (b) from wild-type and Rev-erbα knockout mice exposed to cold for 6 h as described in Fig. 3a, b. c, mRNA levels in preadipocytes isolated from wild-type mice, differentiated in culture and collected at the indicated times after synchronization by serum shock (n = 4). **P < 0.01, ***P < 0.001 as determined by one-way ANOVA with multiple comparisons and a Tukey’s post-test. Data are expressed as mean ± s.d.
Extended Data Figure 5 Rev-erbα controls circadian oscillation of surface temperature and BAT activity.
a, Infrared images from the thermographic surface temperature analysis performed in Fig. 4c. b, Genotypic differences between BAT and core temperatures from wild-type and Rev-erbα knockout mice acclimated to thermoneutrality (n = 6). c, 18FDG imaging (n = 4) of Rev-erbα knockout mice and wild-type littermates during the light and dark phases. Representative sagittal planes are shown for each group. *P < 0.05, Δcore temperature versus ΔBAT temperature; †P < 0.05, core temperature versus Rev-erbα knockout core temperature; ‡P < 0.001, wild-type BAT temperature versus Rev-erbα knockout BAT temperature as determined by Student’s t-test. Data are expressed as mean ± s.e.m.
Extended Data Figure 6 The nuclear receptor Rev-erbα controls circadian thermogenic plasticity.
Rev-erbα regulates the circadian rhythm of body temperature through direct suppression of thermogenesis and BAT activity. Cold exposure during the light phase rapidly overrides Rev-erbα-dependent repression to induce thermogenic programs.
Rights and permissions
About this article
Cite this article
Gerhart-Hines, Z., Feng, D., Emmett, M. et al. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature 503, 410–413 (2013). https://doi.org/10.1038/nature12642
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12642
This article is cited by
-
Brain nuclear receptors and cardiovascular function
Cell & Bioscience (2023)
-
A subpopulation of lipogenic brown adipocytes drives thermogenic memory
Nature Metabolism (2023)
-
Lipolysis regulates major transcriptional programs in brown adipocytes
Nature Communications (2022)
-
Circadian Regulation and Clock-Controlled Mechanisms of Glycerophospholipid Metabolism from Neuronal Cells and Tissues to Fibroblasts
Molecular Neurobiology (2022)
-
Excessive fat expenditure in cachexia is associated with dysregulated circadian rhythm: a review
Nutrition & Metabolism (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.