Abstract
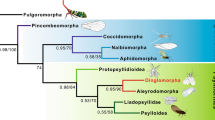
The Eumetabola (Endopterygota (also known as Holometabola) plus Paraneoptera)1 have the highest number of species of any clade, and greatly contribute to animal species biodiversity2,3. The palaeoecological circumstances that favoured their emergence and success remain an intriguing question3,4,5,6. Recent molecular phylogenetic analyses have suggested a wide range of dates for the initial appearance of the Holometabola, from the Middle Devonian epoch (391 million years (Myr) ago) to the Late Pennsylvanian epoch (311 Myr ago7,8,9,10,11,12), and Hemiptera (310 Myr ago13). Palaeoenvironments greatly changed over these periods, with global cooling and increasing complexity of green forests14. The Pennsylvanian-period crown-eumetabolan fossil record remains notably incomplete15,16,17,18,19, particularly as several fossils have been erroneously considered to be stem Holometabola1,15,20,21 (Supplementary Information); the earliest definitive beetles are from the start of the Permian period21,22. The emergence of the hymenopterids, sister group to other Holometabola, is dated between 350 and 309 Myr ago8,9,12, incongruent with their current earliest record (Middle Triassic epoch)1,20. Here we describe five fossils— a Gzhelian-age stem coleopterid, a holometabolous larva of uncertain ordinal affinity, a stem hymenopterid, and early Hemiptera and Psocodea, all from the Moscovian age—and reveal a notable penecontemporaneous breadth of early eumetabolan insects. These discoveries are more congruent with current hypotheses of clade divergence. Eumetabola experienced episodes of diversification during the Bashkirian–Moscovian and the Kasimovian–Gzhelian ages. This cladogenetic activity is perhaps related to notable episodes of drying resulting from glaciations, leading to the eventual demise in Euramerica of coal-swamp ecosystems, evidenced by floral turnover during this interval23,24. These ancient species were of very small size, living in the shadow of Palaeozoic-era ‘giant’ insects. Although these discoveries reveal unexpected Pennsylvanian eumetabolan diversity, the lineage radiated more successfully only after the mass extinctions at the end of the Permian period, giving rise to the familiar crown groups of their respective clades.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Grimaldi, D. A. & Engel, M. S. Evolution of the Insects. (Cambridge Univ. Press, 2005)
Wiegmann, B. M. & Kim, J.-W. in The Timetree of Life (eds Hedges, S. B. & Kumar, S. ) 260–263 (Oxford University Press, 2009)
Mayhew, P. J. Why are there so many insect species? Perspectives from fossils and phylogenies. Biol. Rev. Camb. Philos. Soc. 82, 425–454 (2007)
Bernays, E. A. Evolutionary contrasts in insects: nutritional advantages of holometabolous development. Physiol. Entomol. 11, 377–382 (1986)
Yang, A. S. Modularity, evolvability, and adaptive radiations: a comparison of the hemi- and holometabolous insects. Evol. Dev. 3, 59–72 (2001)
McPeek, M. A. & Brown, J. M. Clade age and not diversification rate explains species richness among animal taxa. Am. Nat. 169, E97–E106 (2007)
Rehm, P. et al. Dating the arthropod tree based on large-scale transcriptome data. Mol. Phylogenet. Evol. 61, 880–887 (2011)
Warnock, R. C. M., Yang, Z. & Donoghue, P. C. J. Exploring uncertainty in the calibration of the molecular clock. Biol. Lett. 8, 156–159 (2012)
Wiegmann, B. M. et al. Single-copy nuclear genes resolve the phylogeny of the holometabolous insects. BMC Biol. 7, 34 (2009)
Yeates, D. K., Cameron, S. L. & Trautwein, M. A view from the edge of the forest: recent progress in understanding the relationships of the insect orders. Aust. J. Entomol. 51, 79–87 (2012)
Wheat, C. W. & Wahlberg, N. Phylogenomic insights into the Cambrian explosion, the colonization of land and the evolution of flight in Arthropoda. Syst. Biol. 62, 93–109 (2013)
Ronquist, F. et al. A total-evidence approach to dating with fossils, applied to the early radiation of the Hymenoptera. Syst. Biol. 61, 973–999 (2012)
Song, N. & Liang, A.-P. A preliminary molecular phylogeny of planthoppers (Hemiptera: Fulgoroidea) based on nuclear and mitochondrial DNA sequences. PLoS ONE 8, e58400 (2013)
Fiz-Palacios, O., Schneider, H., Heinrichs, J. & Savolainen, V. Diversification of land plants: insights from a family-level phylogenetic analysis. BMC Evol. Biol. 11, 341 (2011)
Nel, A. et al. Traits and evolution of wing venation pattern in paraneopteran insects. J. Morphol. 273, 480–506 (2012)
Nel, A. et al. The earliest holometabolous insect: a “crucial” innovation with delayed success (Insecta Protomeropina Protomeropidae). Ann. Soc. Entomol. Fr. (NS) 43, 349–355 (2007)
Nel, P. et al. From Carboniferous to recent: wing venation enlightens evolution of thysanopteran lineage. J. Syst. Palaeontology 10, 385–399 (2012)
Labandeira, C. C. Evidence for an earliest Late Carboniferous divergence time and the early larval ecology and diversification of major Holometabola lineages. Entomol. Amer. 117, 9–21 (2011)
Ilger, J.-M. & Brauckmann, C. The smallest Neoptera (Baryshnyalidae fam. n.) from Hagen-Vorhalle (early Late Carboniferous: Namurian B; Germany). ZooKeys 130, 91–102 (2011)
Rasnitsyn, A. P. & Quicke, D. L. J. History of Insects (Kluwer, 2002)
Kukalová-Peck, J. & Beutel, R. G. Is the Carboniferous †Adiphlebia lacoana really the “oldest beetle”? Critical reassessment and description of a new Permian beetle family. Eur. J. Entomol. 109, 633–645 (2012)
Kirejtshuk, A. G. et al. Evolution of the elytral venation and structural adaptations in the oldest Palaeozoic beetles (Insecta: Coleoptera: Tshekardocoleidae). J. Syst. Palaeontology (in the press)
Davydov, V. I., Korn, D. & Schmitz, M. D. in The Geologic Time Scale (eds Gradstein, F., Ogg, J., Schmitz, M. & Ogg, G. ) 603–651 (Elsevier, 2012)
Phillips, T. L. & Peppers, R. A. Changing patterns of Pennsylvanian coal-swamp vegetation and implications of climatic control on coal occurrence. Int. J. Coal Geol. 3, 205–255 (1984)
Garrouste, R. et al. A complete insect from the Late Devonian period. Nature 488, 82–85 (2012)
Kukalová-Peck, J. & Lawrence, J. F. Relationships among coleopteran suborders and major endoneopteran lineages: evidence from hind wing characters. Eur. J. Entomol. 101, 95–144 (2004)
Beckemeyer, R. J. & Hall, J. D. The entomofauna of the Lower Permian fossil insect beds of Kansas and Oklahoma, USA. Afr. Invertebr. 48, 23–39 (2007)
Hone, D. W. E. & Benton, M. J. The evolution of large size: how does Cope’s Rule work? Trends Ecol. Evol. 20, 4–6 (2005)
Chown, S. L. & Gaston, K. J. Body size variation in insects: a macroecological perspective. Biol. Rev. Camb. Philos. Soc. 85, 139–169 (2010)
Clapham, M. E. & Karr, J. A. Environmental and biotic controls on the evolutionary history of insect body size. Proc. Natl Acad. Sci. USA 109, 10927–10930 (2012)
Acknowledgements
We thank C. C. Labandeira for comments on the first version of the manuscript that helped to improve the paper. We are grateful to A. P. Rasnitsyn, S. I. Golovach and B. R. Striganova, M. Fikáček, A.A. Przhiboro, R. Beutel, T. Hörnschemeyer and V. Krassilov for early discussions. C. Garrouste and P. A. Kirejtshuk assisted in the preparation of illustrations for this publication. Financial support was provided by the Grant Agency of the Czech Republic no. P210/10/0633 (to J.P.) and the German Science Foundation WA 1492/6-1 (to T.W.). The study was supported by the program for visiting researchers and professors of the Smithsonian Institution National Museum of Natural History (NMNH) and partly carried out within the framework of the program of the Presidium of the Russian Academy of Sciences ‘Problems of the origin of life and formation of the biosphere’. A.A.P. and A.G.K. were supported by the Russian Foundation of Basic Research (grant 12-04-00663-a). This paper is a participation to the team project ‘Biodiversity: Origin, Structure, Evolution and Geology’ allotted to D.A. by the Lebanese University.
Author information
Authors and Affiliations
Contributions
A.N., P.R., P.N., A.A.P., T.B., J.P., J.S., D.A., R.G., D.C., D.H., M.S.E. and A.G.K. participated in morphological studies and prepared the manuscript. P.N. and D.C. prepared the figures. P.R. discovered the fossils. The authors of the taxonomic data are associated with the names of the species in the Supplementary Information. A.N. designed the program. M.S.E. and A.G.K. are last authors with equal rank.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
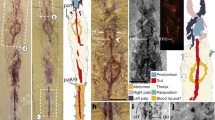
Extended Data Figure 1 Representative of the stem hymenopterids, Avioxyela gallica gen. et sp. nov.
a, Photograph of counterpart. b, Reconstruction of forewing based on part and counterpart. CuA, cubitus anterior; CuP, cubitus posterior; MA, median anterior; MP, median posterior; R, radius; RA, radius anterior; RP, radius posterior; ScP, subcosta posterior. Scale bar, 1 mm (a, b).
Extended Data Figure 2 Representative of the stem hymenopterids, Avioxyela gallica gen. et sp. nov., electron scanning photograph.
a, Two wings partly overlapping. b, Reconstruction of the two forewings. c, Mirror of the second wing to show the identity of pattern with the first wing. d, e, Electron scanning photograph of radial vein of second wing with interpretation of the veins. Scale bar, 1 mm (a, b, c).
Extended Data Figure 3 Representative of the stem coleopterids, Stephanastus polinae gen. et sp. nov.
Electron scanning photographs. a, Foreleg and pronotum. b, Mid-leg. Scale bar, 1 mm (a, b).
Extended Data Figure 4 Holometabolous larva, Metabolarva bella gen. et sp. nov.
Photograph of posterior half of body. Scale bar, 1 mm.
Extended Data Figure 5 Representative of stem Euhemiptera, Aviorrhyncha magnifica gen. et sp. nov.
a, Photograph of part. b, Electron scanning photograph of counterpart. AA1 + 2, first anal anterior vein; AA3 + 4, second anal anterior vein; PC, precosta. Scale bar, 1 mm (a, b).
Extended Data Figure 6 Euhemiptera wing venation.
a, Wing base of Aviorrhyncha magnifica gen. et sp. nov. b, Wing base of a modern Fulgoroidea. Scale bar, 1 mm (a, b).
Extended Data Figure 7 Representative of stem Psocodea, Westphalopsocus pumilio gen. et sp. nov.
a, Reconstruction of forewing. b, Photograph. A, anal veins. Scale bar, 1 mm (a, b).
Supplementary information
Supplementary Information
This file contains Supplementary Text and Supplementary References. (PDF 514 kb)
Rights and permissions
About this article
Cite this article
Nel, A., Roques, P., Nel, P. et al. The earliest known holometabolous insects. Nature 503, 257–261 (2013). https://doi.org/10.1038/nature12629
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12629
This article is cited by
-
First Eugereonidae (Insecta: Palaeodictyoptera) from the Pennsylvanian (Late Carboniferous) of the Piesberg site near Osnabrück, Germany
PalZ (2023)
-
South African Lagerstätte reveals middle Permian Gondwanan lakeshore ecosystem in exquisite detail
Communications Biology (2022)
-
Debris-carrying behaviour of bark lice immatures preserved in 100 million years old amber
PalZ (2022)
-
New extreme morphologies as exemplified by 100 million-year-old lacewing larvae
Scientific Reports (2021)
-
Gene content evolution in the arthropods
Genome Biology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.