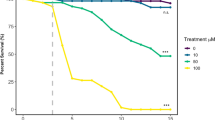
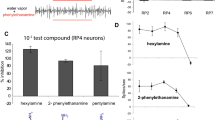
Abstract
There are major impediments to finding improved DEET alternatives because the receptors causing olfactory repellency are unknown, and new chemicals require exorbitant costs to determine safety for human use. Here we identify DEET-sensitive neurons in a pit-like structure in the Drosophila melanogaster antenna called the sacculus. They express a highly conserved receptor, Ir40a, and flies in which these neurons are silenced or Ir40a is knocked down lose avoidance to DEET. We used a computational structure–activity screen of >400,000 compounds that identified >100 natural compounds as candidate repellents. We tested several and found that most activate Ir40a+ neurons and are repellents for Drosophila. These compounds are also strong repellents for mosquitoes. The candidates contain chemicals that do not dissolve plastic, are affordable and smell mildly like grapes, with three considered safe in human foods. Our findings pave the way to discover new generations of repellents that will help fight deadly insect-borne diseases worldwide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Krajick, K. Medical entomology–Keeping the bugs at bay. Science 313, 36–38 (2006)
Corbel, V. et al. Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent deet. BMC Biol. 7, 47 (2009)
Reeder, N. L., Ganz, P. J., Carlson, J. R. & Saunders, C. W. Isolation of a DEET-insensitive mutant of Drosophila melanogaster (Diptera: Drosophilidae). J. Econ. Entomol. 94, 1584–1588 (2001)
Klun, J. A. et al. Comparative resistance of Anopheles albimanus and Aedes aegypti to N,N-diethyl-3-methylbenzamide (Deet) and 2-methylpiperidinyl-3-cyclohexen-1-carboxamide (AI3–37220) in laboratory human-volunteer repellent assays. J. Med. Entomol. 41, 418–422 (2004)
Stanczyk, N. M., Brookfield, J. F., Ignell, R., Logan, J. G. & Field, L. M. Behavioral insensitivity to DEET in Aedes aegypti is a genetically determined trait residing in changes in sensillum function. Proc. Natl Acad. Sci. USA 107, 8575–8580 (2010)
Gupta, R. K. & Bhattacharjee A. K in Insect Repellents: Principles, Methods, and Uses (eds M. Debboun, Frances, S.P. & Strickman, D. ) 195–228 (Taylor & Francis Group, 2007)
Ditzen, M., Pellegrino, M. & Vosshall, L. B. Insect odorant receptors are molecular targets of the insect repellent DEET. Science 319, 1838–1842 (2008)
Bohbot, J. D. & Dickens, J. C. Odorant receptor modulation: ternary paradigm for mode of action of insect repellents. Neuropharmacology 62, 2086–2095 (2012)
Pellegrino, M., Steinbach, N., Stensmyr, M. C., Hansson, B. S. & Vosshall, L. B. A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor. Nature 478, 511–514 (2011)
Syed, Z. & Leal, W. S. Mosquitoes smell and avoid the insect repellent DEET. Proc. Natl Acad. Sci. USA 105, 13598–13603 (2008)
DeGennaro, M. et al. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498, 487–491 (2013)
Xia, Y. et al. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc. Natl Acad. Sci. USA 105, 6433–6438 (2008)
Liu, C. et al. Distinct olfactory signaling mechanisms in the malaria vector mosquito Anopheles gambiae. PLoS Biol. 8, (2010)
Lee, Y., Kim, S. H. & Montell, C. Avoiding DEET through insect gustatory receptors. Neuron 67, 555–561 (2010)
Weiss, L. A., Dahanukar, A., Kwon, J. Y., Banerjee, D. & Carlson, J. R. The molecular and cellular basis of bitter taste in Drosophila. Neuron 69, 258–272 (2011)
Masuyama, K., Zhang, Y., Rao, Y. & Wang, J. W. Mapping neural circuits with activity-dependent nuclear import of a transcription factor. J. Neurogenet. 26, 89–102 (2012)
Syed, Z., Pelletier, J., Flounders, E., Chitolina, R. F. & Leal, W. S. Generic insect repellent detector from the fruit fly Drosophila melanogaster. PLoS ONE 6, e17705 (2011)
Silbering, A. F. et al. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 31, 13357–13375 (2011)
Ai, M. et al. Acid sensing by the Drosophila olfactory system. Nature 468, 691–695 (2010)
Benton, R., Vannice, K. S., Gomez-Diaz, C. & Vosshall, L. B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162 (2009)
Abuin, L. et al. Functional architecture of olfactory ionotropic glutamate receptors. Neuron 69, 44–60 (2011)
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods 6, 875–881 (2009)
Pelz, D., Roeske, T., Syed, Z., de Bruyne, M. & Galizia, C. G. The molecular receptive range of an olfactory receptor in vivo (Drosophila melanogaster Or22a). J. Neurobiol. 66, 1544–1563 (2006)
Sweeney, S. T., Broadie, K., Keane, J., Niemann, H. & O'Kane, C. J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14, 341–351 (1995)
Katritzky, A. R. et al. Synthesis and bioassay of improved mosquito repellents predicted from chemical structure. Proc. Natl Acad. Sci. USA 105, 7359–7364 (2008)
Klocke, J. A., Darlington, M. V. & Balandrin, M. F. 1,8-Cineole (Eucalyptol), a mosquito feeding and ovipositional repellent from volatileoil of Hemizonia fitchii (Asteraceae). J. Chem. Ecol. 13, 2131–2141 (1987)
Kline, D. L., Bernier, U. R., Posey, K. H. & Barnard, D. R. Olfactometric evaluation of spatial repellents for Aedes aegypti. J. Med. Entomol. 40, 463–467 (2003)
Carey, A. F., Wang, G., Su, C. Y., Zwiebel, L. J. & Carlson, J. R. Odorant reception in the malaria mosquito Anopheles gambiae. Nature 464, 66–71 (2010)
Hallem, E. A. & Carlson, J. R. Coding of odors by a receptor repertoire. Cell 125, 143–160 (2006)
Haddad, R. et al. A metric for odorant comparison. Nature Methods 5, 425–429 (2008)
Cortes, C. & Vapnik, V. Support-vector networks. Mach. Learn. 20, 273–297 (1995)
Walker, J. D., Rodford, R. & Patlewicz, G. Quantitative structure–activity relationships for predicting percutaneous absorption rates. Environ. Toxicol. Chem. 22, 1870–1884 (2003)
Tentschert, J., Bestmann, H. J., Holldobler, B. & Heinze, J. 2,3-dimethyl-5-(2-methylpropyl)pyrazine, a trail pheromone component of Eutetramorium mocquerysi Emery (1899) (Hymenoptera: Formicidae). Naturwissenschaften 87, 377–380 (2000)
Mumcuoglu, K. Y., Galun, R., Bach, U., Miller, J. & Magdassi, S. Repellency of essential oils and their components to the human body louse, Pediculus humanus humanus. Entomol. Exp. Appl. 78, 309–314 (1996)
Hou, X. W., Fields, P. & Taylor, W. The effect of repellents on penetration into packaging by stored-product insects. J. Stored Prod. Res. 40, 47–54 (2004)
Abramson, C. I. et al. Proboscis conditioning experiments with honeybees, Apis mellifera caucasica, with butyric acid and DEET mixture as conditioned and unconditioned stimuli. J. Insect Sci. 10, 1–17 (2010)
Turner, S. L. & Ray, A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature 461, 277–281 (2009)
Turner, S. L. et al. Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature 474, 87–91 (2011)
de Bruyne, M., Clyne, P. J. & Carlson, J. R. Odor coding in a model olfactory organ: the Drosophila maxillary palp. J. Neurosci. 19, 4520–4532 (1999)
Boström, J., Greenwood, J. R. & Gottfries, J. Assessing the performance of OMEGA with respect to retrieving bioactive conformations. J. Mol. Graph Model. 21, 449–462 (2003)
DRAGON. software for Windows for molecular descriptor calculations v.5.5 (Talete, 2007)
Chang, C. & Lin, C. Libsvm: a library for support vector machines. (http://www.csie.ntu.edu.tw/∼cjlin/libsvm) (2001)
Karatzoglou, A., Meyer, D. & Hornik, K. Support vector machines in R. J. Stat. Softw. http://www.jstatsoft.org/v15/i09 (2006)
El-Sayed, A. M. The Pherobase database of insect pheromones and semiochemicals http://www.pherobase.com/ (2009)
Flavors. and fragrances 2007–2008 catalogue (Sigma-Aldrich, 2007)
Cork, A. & Park, K. C. Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med. Vet. Entomol. 10, 269–276 (1996)
Curran, A. M., Rabin, S. I., Prada, P. A. & Furton, K. G. Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J. Chem. Ecol. 31, 1607–1619 (2005)
Gallagher, M. et al. Analyses of volatile organic compounds from human skin. Br. J. Dermatol. 159, 780–791 (2008)
Knudsen, J. T., Eriksson, R., Gershenzon, J. & Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 72, 1–120 (2006)
Logan, J. G. et al. Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J. Chem. Ecol. 34, 308–322 (2008)
Meijerink, J. et al. Identification of olfactory stimulants for Anopheles gambiae from human sweat samples. J. Chem. Ecol. 26, 1367–1382 (2000)
Zeng, X. N. et al. Analysis of characteristic odors from human male axillae. J. Chem. Ecol. 17, 1469–1492 (1991)
Zeng, X. N., Leyden, J. J., Spielman, A. I. & Preti, G. Analysis of characteristic human female axillary odors: qualitative comparison to males. J. Chem. Ecol. 22, 237–257 (1996)
Acknowledgements
We thank A. Ganguly and D. Carter for help with calcium imaging; Z. Wisotsky for help with gustatory experiments; D. MacWilliam for help with olfactory experiments; J. Wang for sharing the NFAT transgenic fly line; and R. Benton for sharing the Ir40a-Gal4 fly line. This work was partly funded by a Whitehall Foundation grant to A.D., an R21NS074332 (NINDS) to A.D. and A.R., and an R56AI099778 (NIAID) and R01AI087785 (NIAID) to A.R. The granting agencies had no role in experimental design or analysis.
Author information
Authors and Affiliations
Contributions
S.M.B. planned and performed the chemical informatics and solubility experiment, and helped design the behaviour experiments. P.K. planned and performed the NFAT imaging, Ca2+ imaging and Drosophila behaviour experiments. S.K.T. performed Ca2+ imaging, electrophysiology and some behaviour experiments. T.G. performed the arm-in-cage experiments. C.P. performed behaviour analysis. S.M.B., P.K. and S.K.T. helped prepare drafts of the manuscript and figures. A.D. planned experiments and helped write the manuscript. A.R. planned experiments, managed the project, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
P.K., S.M.B., C.P. and A.R. are listed as inventors in pending patent applications filed by the University of California Riverside.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-11 and Supplementary Tables 1-2. (PDF 2933 kb)
DEET activates neurons in the sacculus
This video shows a confocal Z-stack of a representative antenna from LexAop-CD8-GFP-2ACD8-GFP; UAS-mLexA-VP16-NFAT, LexAop-CD2-GFP D. melanogaster exposed to10% DEET (from experiment in Figure 1b). (AVI 505 kb)
BA activates neurons in the sacculus
This video shows a confocal Z-stack of a representative antenna from LexAop-CD8-GFP-2ACD8-GFP; UAS-mLexA-VP16-NFAT, LexAop-CD2-GFP D. melanogaster exposed to 10% BA (from experiment in Figure 5a). (AVI 1436 kb)
Rights and permissions
About this article
Cite this article
Kain, P., Boyle, S., Tharadra, S. et al. Odour receptors and neurons for DEET and new insect repellents. Nature 502, 507–512 (2013). https://doi.org/10.1038/nature12594
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12594
This article is cited by
-
An odorant receptor mediates the avoidance of Plutella xylostella against parasitoid
BMC Biology (2024)
-
Toxicity, repellency and chemical composition of essential oils from Cymbopogon species against red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae)
Journal of Consumer Protection and Food Safety (2020)
-
Insecticide resistance modifies mosquito response to DEET and natural repellents
Parasites & Vectors (2019)
-
Candidate chemosensory genes identified from the greater wax moth, Galleria mellonella, through a transcriptomic analysis
Scientific Reports (2019)
-
An odorant receptor from Anopheles sinensis in China is sensitive to oviposition attractants
Malaria Journal (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.