Abstract
Replication fork maintenance pathways preserve chromosomes, but their faulty application at nonallelic repeats could generate rearrangements causing cancer, genomic disorders and speciation1,2,3. Potential causal mechanisms are homologous recombination and error-free postreplication repair (EF-PRR). Homologous recombination repairs damage-induced DNA double-strand breaks (DSBs) and single-ended DSBs within replication. To facilitate homologous recombination, the recombinase RAD51 and mediator BRCA2 form a filament on the 3′ DNA strand at a break to enable annealing to the complementary sister chromatid4 while the RecQ helicase, BLM (Bloom syndrome mutated) suppresses crossing over to prevent recombination5. Homologous recombination also stabilizes6,7 and restarts8,9 replication forks without a DSB10,11. EF-PRR bypasses DNA incongruities that impede replication by ubiquitinating PCNA (proliferating cell nuclear antigen) using the RAD6–RAD18 and UBC13–MMS2–RAD5 ubiquitin ligase complexes12. Some components are common to both homologous recombination and EF-PRR such as RAD51 and RAD1813,14. Here we delineate two pathways that spontaneously fuse inverted repeats to generate unstable chromosomal rearrangements in wild-type mouse embryonic stem (ES) cells. Gamma-radiation induced a BLM-regulated pathway that selectively fused identical, but not mismatched, repeats. By contrast, ultraviolet light induced a RAD18-dependent pathway that efficiently fused mismatched repeats. Furthermore, TREX2 (a 3′→5′ exonuclease) suppressed identical repeat fusion but enhanced mismatched repeat fusion, clearly separating these pathways. TREX2 associated with UBC13 and enhanced PCNA ubiquitination in response to ultraviolet light, consistent with it being a novel member of EF-PRR. RAD18 and TREX2 also suppressed replication fork stalling in response to nucleotide depletion. Interestingly, replication fork stalling induced fusion for identical and mismatched repeats, implicating faulty replication as a causal mechanism for both pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hastings, P. J., Lupski, J. R., Rosenberg, S. M. & Ira, G. Mechanisms of change in gene copy number. Nature Rev. Genet. 10, 551–564 (2009)
Carr, A. M., Paek, A. L. & Weinert, T. DNA replication: failures and inverted fusions. Semin. Cell Dev. Biol. 22, 866–874 (2011)
Lee, J. A., Carvalho, C. M. & Lupski, J. R. A. DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell 131, 1235–1247 (2007)
San Filippo, J., Sung, P. & Klein, H. Mechanism of eukaryotic homologous recombination. Annu. Rev. Biochem. 77, 229–257 (2008)
Wu, L. & Hickson, I. D. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature 426, 870–874 (2003)
Schlacher, K. et al. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell 145, 529–542 (2011)
Schlacher, K., Wu, H. & Jasin, M. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell 22, 106–116 (2012)
Mizuno, K., Lambert, S., Baldacci, G., Murray, J. M. & Carr, A. M. Nearby inverted repeats fuse to generate acentric and dicentric palindromic chromosomes by a replication template exchange mechanism. Genes Dev. 23, 2876–2886 (2009)
Mizuno, K., Miyabe, I., Schalbetter, S. A., Carr, A. M. & Murray, J. M. Recombination-restarted replication makes inverted chromosome fusions at inverted repeats. Nature 493, 246–249 (2013)
Petermann, E., Orta, M. L., Issaeva, N., Schultz, N. & Helleday, T. Hydroxyurea-stalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair. Mol. Cell 37, 492–502 (2010)
Carr, A. M. & Lambert, S. Replication stress-induced genome instability: the dark side of replication maintenance by homologous recombination. J. Mol. Biol.. http://dx.doi.org/10.1016/j.jmb.2013.04.023 (30 April 2013)
Ulrich, H. D. Regulating post-translational modifications of the eukaryotic replication clamp PCNA. DNA Repair (Amst.) 8, 461–469 (2009)
Falbo, K. B. et al. Involvement of a chromatin remodeling complex in damage tolerance during DNA replication. Nature Struct. Mol. Biol. 16, 1167–1172 (2009)
Huang, J. et al. RAD18 transmits DNA damage signalling to elicit homologous recombination repair. Nature Cell Biol. 11, 592–603 (2009)
Kim, T. M. et al. RAD51 mutants cause replication defects and chromosomal instability. Mol. Cell. Biol. 32, 3663–3680 (2012)
Shimizu, N., Shingaki, K., Kaneko-Sasaguri, Y., Hashizume, T. & Kanda, T. When, where and how the bridge breaks: anaphase bridge breakage plays a crucial role in gene amplification and HSR generation. Exp. Cell Res. 302, 233–243 (2005)
Cavalli, G. & Misteli, T. Functional implications of genome topology. Nature Struct. Mol. Biol. 20, 290–299 (2013)
Harada, S., Sekiguchi, N. & Shimizu, N. Amplification of a plasmid bearing a mammalian replication initiation region in chromosomal and extrachromosomal contexts. Nucleic Acids Res. 39, 958–969 (2011)
Horvath, J. E. et al. Using a pericentromeric interspersed repeat to recapitulate the phylogeny and expansion of human centromeric segmental duplications. Mol. Biol. Evol. 20, 1463–1479 (2003)
Luo, G. et al. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nature Genet. 26, 424–429 (2000)
Motegi, A. et al. Polyubiquitination of proliferating cell nuclear antigen by HLTF and SHPRH prevents genomic instability from stalled replication forks. Proc. Natl Acad. Sci. USA 105, 12411–12416 (2008)
Tateishi, S. et al. Enhanced genomic instability and defective postreplication repair in RAD18 knockout mouse embryonic stem cells. Mol. Cell. Biol. 23, 474–481 (2003)
Chen, M. J., Ma, S. M., Dumitrache, L. C. & Hasty, P. Biochemical and cellular characteristics of the 3′ → 5′ exonuclease TREX2. Nucleic Acids Res. 35, 2682–2694 (2007)
Dumitrache, L. C., Hu, L. & Hasty, P. TREX2 exonuclease defective cells exhibit double-strand breaks and chromosomal fragments but not Robertsonian translocations. Mutat. Res. 662, 84–87 (2009)
Chen, M. J. et al. Cisplatin depletes TREX2 and causes Robertsonian translocations as seen in TREX2 knockout cells. Cancer Res. 67, 9077–9083 (2007)
Dumitrache, L. C. et al. Trex2 enables spontaneous sister chromatid exchanges without facilitating DNA double-strand break repair. Genetics 188, 787–797 (2011)
Goldfless, S. J., Morag, A. S., Belisle, K. A., Sutera, V. A., Jr & Lovett, S. T. DNA repeat rearrangements mediated by DnaK-dependent replication fork repair. Mol. Cell 21, 595–604 (2006)
Dutra, B. E. & Lovett, S. T. Cis and trans-acting effects on a mutational hotspot involving a replication template switch. J. Mol. Biol. 356, 300–311 (2006)
Sirbu, B. M. et al. Analysis of protein dynamics at active, stalled, and collapsed replication forks. Genes Dev. 25, 1320–1327 (2011)
Blastyák, A., Hajdú, I., Unk, I. & Haracska, L. Role of double-stranded DNA translocase activity of human HLTF in replication of damaged DNA. Mol. Cell. Biol. 30, 684–693 (2010)
Reid, L. H., Gregg, R. G., Smithies, O. & Koller, B. H. Regulatory elements in the introns of the human HPRT gene are necessary for its expression in embryonic stem cells. Proc. Natl Acad. Sci. USA 87, 4299–4303 (1990)
Adra, C. N., Boer, P. H. & McBurney, M. W. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene 60, 65–74 (1987)
Guenatri, M., Bailly, D., Maison, C. & Almouzni, G. Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J. Cell Biol. 166, 493–505 (2004)
Montagna, C., Andrechek, E. R., Padilla-Nash, H., Muller, W. J. & Ried, T. Centrosome abnormalities, recurring deletions of chromosome 4, and genomic amplification of HER2/neu define mouse mammary gland adenocarcinomas induced by mutant HER2/neu. Oncogene 21, 890–898 (2002)
Davisson, M. T. Rules and guidelines for nomenclature of mouse genes. Gene 147, 157–160 (1994)
Holcomb, V. B. et al. HPRT minigene generates chimeric transcripts as a by-product of gene targeting. Genesis 45, 275–281 (2007)
Ramírez-Solis, R. et al. Genomic DNA microextraction: a method to screen numerous samples. Anal. Biochem. 201, 331–335 (1992)
Soriano, P., Montgomery, C., Geske, R. & Bradley, A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702 (1991)
Kim, D. H. et al. The CRL4Cdt2 ubiquitin ligase mediates the proteolysis of cyclin-dependent kinase inhibitor Xic1 through a direct association with PCNA. Mol. Cell. Biol. 30, 4120–4133 (2010)
Krijger, P. H. et al. HLTF and SHPRH are not essential for PCNA polyubiquitination, survival and somatic hypermutation: existence of an alternative E3 ligase. DNA Repair (Amst.) 10, 438–444 (2011)
Friedrich, G. & Soriano, P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5, 1513–1523 (1991)
Araki, K., Araki, M. & Yamamura, K. Targeted integration of DNA using mutant lox sites in embryonic stem cells. Nucleic Acids Res. 25, 868–872 (1997)
Kim, T. M., Choi, Y. J., Ko, J. H. & Hasty, P. High-throughput knock-in coupling gene targeting with the HPRT minigene and Cre-mediated recombination. Genesis 46, 732–737 (2008)
Donoho, G. et al. Deletion of Brca2 exon 27 causes hypersensitivity to DNA crosslinks, chromosomal instability, and reduced life span in mice. Genes Chromosom. Cancer 36, 317–331 (2003)
Morimatsu, M., Donoho, G. & Hasty, P. Cells deleted for Brca2 COOH terminus exhibit hypersensitivity to gamma- radiation and premature senescence. Cancer Res. 58, 3441–3447 (1998)
Moynahan, M. E., Pierce, A. J. & Jasin, M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 7, 263–272 (2001)
Terai, K., Abbas, T., Jazaeri, A. A. & Dutta, A. CRL4Cdt2 E3 ubiquitin ligase monoubiquitinates PCNA to promote translesion DNA synthesis. Mol. Cell 37, 143–149 (2010)
Acknowledgements
We thank C. Williams and S. Dodds for technical assistance and the Molecular Cytogenetic Core at Albert Einstein College of Medicine for help with the execution of spectral karyotyping and two-colour FISH. This work was supported by the National Institutes of Health (1 RO1 CA123203-01A1 to P.H., 2P01AG017242-12 to P.H. and C.M. P30CA013330 to C.M.) and with support from the Cancer Therapy & Research Center at The University of Texas at San Antonio (CTRC) (P30 CA054174).
Author information
Authors and Affiliations
Contributions
L.H., T.M.K., P.R.Y., C.M., L.C.D. and P.H. designed experiments and interpreted results. L.H., T.M.K., M.Y.S., S.-AK., C.L.H., D.H.K. and PH performed experiments. S.T. provided the rad18−/− and IB10 ES cells. P.H. wrote the paper with comments from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
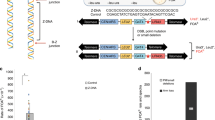
Extended Data Figure 1 Three locations for the switch within a hairpin.
There are seven mismatches located at positions 52, 111, 140, 178, 188, 204 and 246. This model shows the inverted repeats forming a hairpin to simply illustrate the location of the switch, although we do not know if hairpins form. a, The switch occurs at the apex of the hairpin before the first mismatch at position 52 such that the 5′ MSR has the same sequence as the orange repeat. b, The switch occurs in the stem of the hairpin after the first mismatch at position 52 but before the last mismatch at position 246 such that the 5′ MSR is a mixture of both the green and orange repeat. c, The switch occurs at the base of the hairpin after the last mismatch in position 246 such that the 5′ MSR has the same sequence as the green repeat.
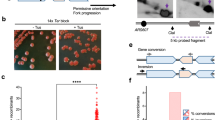
Extended Data Figure 2 Complex chromosomal rearrangements in wild-type cells with the IRR and MRR.
a, Two-colour FISH on metaphase spreads stained with a telomeric probe (green), a MSR probe in the pericentromere (red) and counterstained with DAPI (blue). (1)–(3) Multipericentric chromosomes from cells with the IRR: (1) Typical dipericentric, (2) chromosome with extra pericentromeres and telomeres (EPT)15, (3) segmental duplication with the extra pericentromeres on only one chromatid. (4)–(8) Multipericentric chromosomes from cells with the MRR: (4) typical dipericentric, (5)–(7) EPTs, (8) extra pericentromere on only one chromatid. Chromosomal abnormalities were found for 15/19 (P < 0.0001, Yates-corrected chi-square test) and 18/19 (P < 0.0001) HAT-resistant colonies transfected with the IRR and MRR, respectively, but none were found for non-transfected cells as previously described15. b, Two-colour FISH on nuclei using the MRR as a probe (red) along with either chromosome 1 or 14 (green). For some nuclei the MMR associated with chromosome 14 (1) whereas for others it associated with chromosome 1 (2). Note the MRR is located to both chromosomes 14 but only one chromosome 1. Thus, the MRR moved to different altered chromosomes observed with spectral karyotyping, consistent with the notion that the MRR is the source of instability. In addition, the size of the red dot(s) varied, suggesting continuous nonallelic fusions that could expand or contract the number of MRR units. For some nuclei the MRR appeared as a discrete dot, indicating one contiguous array of reporter units (1 and 2, red insets) but for others it was speckled, suggesting arrays of MRR units were interspersed with chromosomal sequences (3, red inset). For one speckled cluster a fragment of chromosome 1 surrounded only one red dot, highlighting the complexity of this rearrangement (green inset). The MRR probe was also found protruding at the edge or outside of some nuclei, indicating these unstable structures could be extruded from the nucleus similar to micronuclei (4, red inset).
Extended Data Figure 3 Targeting Rad51 exons 2–4.
a, SAβgeo-miniHPRT is used for selection. SAβgeo (green) is a fusion of β-galactosidase and neomycin phosphotransferase and is capable of trapping promoters to improve targeting efficiency41. A Right element (RE) mutant loxP42 is in the intron (blue green arrow). In addition, another RE mutant loxP is 5′ to SAβgeo. A FLP recombination target (FRT) is at the 3′ end of miniHPRT36,43. b, Replacing Rad51 exons 2–4 (exon 2 is the first coding exon) with the SAβgeo-miniHPRT selection cassette. PCR is used to screen G418+HAT-resistant ES cell clones for gene targeting using primers H13F and SR3. c, Removal of SAβgeo, the 5′ half of miniHPRT and a RE mutant loxP by Cre-mediated recombination to generate Rad51+/Δex2-4 cells. Screen 6-thioguanine-resistant clones by PCR using primers RCF1 and AS2.
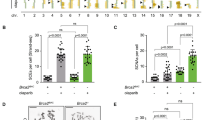
Extended Data Figure 4 Targeting Brca2 exon 27.
There were two gene targeting vectors so we could observe cells deleted for one (blm−/− Brca2+/Δex27-n) and two (blm−/− brca2Δex27-h/Δex27-n) copies of Brca2 exon 27. a, The first targeting vector (Δex27-n) replaced Brca2 exon 27 with neomycin phosphotransferase (neo) and probably generated a severe defect because exon 27 was not replaced with a splice donor to ensure polyadenylation44. This means deletion of the first copy probably caused a haploinsufficiency. The Brca2 gene after targeting. NF and B27R are PCR primers used to screen for targeted clones. b, The second targeting vector (Δex27-h) replaced Brca2 exon 27 with miniHPRT that contains a splice donor and polyadenylation sequences. Previously we showed Brca2 exon 26 spliced into HPRT exon 3 to ensure polyadenylation. Cells mutated with this second targeting vector produced a truncated BRCA2 protein at normal levels and were hypersensitive to γ-radiation and deficient in homologous recombination36,45,46 and replication fork maintenance6. Replacing the second copy of Brca2 exon 27 with a floxed miniHPRT36 to make Brca2Δex27-h/Δex27-n cells. H13F and B27R primers were used to screen for targeted clones. Cre-mediated recombination removed the 5′ half of miniHPRT. Brca2 exon 26 splices into miniHPRT exons 3–8 (grey line) to generate a polyadenylated Brca2 transcript that is deleted for exon 2736,45. There is the addition of one amino acid followed by a stop codon and this transcript produces a protein at wild-type levels that associates with RAD51, presumably through the BRC motifs46. Bi26 and H3-8R PCR primers were used to screen for Cre-mediated deletion.
Extended Data Figure 5 TREX2’s response to ultraviolet light and association with UBC13.
a, Coimmunoprecipitation of IdU and Myc-TREX2 in HeLa cells after exposure to 20 J m−2 ultraviolet light. No treatment, NT. b, GST pull-down of 35S-labelled short isoform wild-type (WT) TREX223. c, Coimmunoprecipitation with Myc-TREX2 and HA-UBC13 in HeLa cells before and 6 h after exposure to 20 J m−2 ultraviolet light.
Extended Data Figure 6 RAD18 and TREX2 ubiquitinate PCNA.
a, Exposure of AB2.2 cells to ultraviolet light, but not γ-radiation, induced PCNA ubiquitination. Immunoprecipitate endogenous PCNA and immunoblot with anti-ubiquitin (Ub, left), then strip and immunoblot with anti-PCNA (right). PCNA–Ub1 and PCNA–Ub3 are visible; yet, IgG obscures PCNA–Ub2. In addition, the Ub blot, but not the PCNA blot, reveals a previously unidentified band between PCNA–Ub1 and PCNA–Ub2. Ultraviolet light, but not γ-radiation, increased levels of PCNA–Ub1 and PCNA–Ub3 as previously shown in human cells21 (the same was true for the unknown protein). Survival fraction: 20 J m−2, 0.6%; 60 J m−2, 0.06%; 5 Gy, 8%; 15 Gy; 0.001%. b, Analysis of trex2null and rad18−/− cells and double-mutant cells. In response to 60 J m−2 ultraviolet light, trex2null and rad18−/− cells had reduced levels of PCNA–Ub1 and PCNA–Ub3 and unknown protein as compared to IB10 cells. rad18−/− cells exhibited a marginally greater reduction than trex2null cells, indicating that RAD18 has a greater role in PCNA ubiquitination. The double-mutant cells failed to show a further reduction, indicating that TREX2 and RAD18 are epistatic. Some ubiquitinated PCNA was present in mutant cells, indicating that other proteins ubiquitinate PCNA; similar observations were made for cells deleted for HLTF and SHPRH40. For example, CRL4Cdt2, independent of RAD18, monoubiquitinates PCNA with and without ultraviolet-light-induced damage47. c, Bar graph illustrating the reduction of PCNA–Ub1 and PCNA–Ub3 in trex2null, rad18−/−, and double-mutant cells as shown in b, left (immunoprecipitation-PCNA, blot-Ub), after band intensities were quantified with ImageJ and normalized for loading with short exposure PCNA. Statistics (t-test) for PCNA–Ub1 and PCNA–Ub3 using three experiments (lanes): 1 vs 2 (0.0016, 0.0058), 1 vs 3 (0.0036, 0.0026), 1 vs 4 (0.0064, 0.0001), 2 vs 3 (0.0214, 0.0774), 2 vs 4 (0.0310, 0.0486), 3 vs 4 (0.3169, 0.1209). d, Bar graph illustrating the reduction of PCNA–Ub1 in trex2null, rad18−/−, and double-mutant cells as shown in b, right (immunoprecipitation-PCNA, blot-PCNA), after band intensities were quantified with ImageJ and normalized for loading with short exposure PCNA. The stripping and re-probing leaves quantification unreliable for PCNA–Ub3 and further work is required to clarify the extent to which Ub modification is influenced in these backgrounds. Statistics (t-test) for PCNA–Ub1 using three experiments (lanes): 1 vs 2 (0.0021), 1 vs 3 (0.0061), 1 vs 4 (0.0460), 2 vs 3 (0.0212), 2 vs 4 (0.0163), 3 vs 4 (0.0604).
Extended Data Figure 7 Deleting Trex2 in IB10 control and rad18−/− cells.
A floxed MiniHPRT36 was used to replace the entire Trex2 coding sequences (located on a single exon)25. Targeted clones were detected using PCR with TX2 LR55 and HATrev primers for the left arm and HATfor and TX2 RR33 primers for the right arm. Removal of the Trex2 coding sequence was verified by PCR using mTX2For and mTX2Rev primers.
Rights and permissions
About this article
Cite this article
Hu, L., Kim, T., Son, M. et al. Two replication fork maintenance pathways fuse inverted repeats to rearrange chromosomes. Nature 501, 569–572 (2013). https://doi.org/10.1038/nature12500
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12500
This article is cited by
-
DNA methylation at an enhancer of the three prime repair exonuclease 2 gene (TREX2) is linked to gene expression and survival in laryngeal cancer
Clinical Epigenetics (2019)
-
Analysis of horse genomes provides insight into the diversification and adaptive evolution of karyotype
Scientific Reports (2014)
-
Causes and consequences of replication stress
Nature Cell Biology (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.