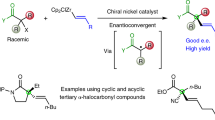
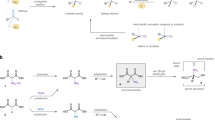
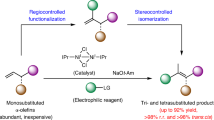
Abstract
The SN2 reaction (bimolecular nucleophilic substitution) is a well-known chemical transformation that can be used to join two smaller molecules together into a larger molecule or to exchange one functional group for another. The SN2 reaction proceeds in a very predictable manner: substitution occurs with inversion of stereochemistry, resulting from the ‘backside attack’ of the electrophilic carbon by the nucleophile. A significant limitation of the SN2 reaction is its intolerance for tertiary carbon atoms: whereas primary and secondary alcohols are viable precursor substrates, tertiary alcohols and their derivatives usually either fail to react or produce stereochemical mixtures of products1,2,3. Here we report the stereochemical inversion of chiral tertiary alcohols with a nitrogenous nucleophile facilitated by a Lewis-acid-catalysed solvolysis. The method is chemoselective against secondary and primary alcohols, thereby complementing the selectivity of the SN2 reaction. Furthermore, this method for carbon–nitrogen bond formation mimics a putative biosynthetic step in the synthesis of marine terpenoids4 and enables their preparation from the corresponding terrestrial terpenes. We expect that the general attributes of the methodology will allow chiral tertiary alcohols to be considered viable substrates for stereoinversion reactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
Data deposits
Crystallographic data are deposited with the Cambridge Crystallographic Data Centre under accession numbers CCDC 949094 (25) and CCDC 948968 (26).
Change history
13 November 2013
A Correction to this paper has been published: https://doi.org/10.1038/nature12795
References
Hughes, E. D. Ingold, C. K., Martin, R. J. L. & Meigh, D. F. Walden inversion and reaction mechanism: Walden inversion in unimolecular reactions of secondary and tertiary alkyl halides. Nature 166, 679–680 (1950)
Bunton, C. A. Hughes, E. D., Ingold, C. K. & Meigh, D. F. Walden inversion and reaction mechanism: Walden inversion in the acid hydrolysis of carboxylic esters by unimolecular alkyl fission. Nature 166, 680 (1950)
Mascal, M. Hafezi, N. & Toney, M. D. 1,4,7-Trimethyloxatriquinane: SN2 reaction at tertiary carbon. J. Am. Chem. Soc. 132, 10662–10664 (2010)
Garson, M. J. &. Simpson, J. S. Marine isocyanides and related natural products—structure, biosynthesis and ecology. Nat. Prod. Rep. 21, 164–179 (2004)
Dewick, P. M. Medicinal Natural Products: A Biosynthetic Approach (Wiley, 2009)
Hagadone, M. R. & Scheuer, P. J. On the origin of the isocyano function in marine sponges. J. Am. Chem. Soc. 106, 2447–2449 (1984)
Rubenbauer, P. & Bach, T. Diastereoselective Ritter reactions of chiral secondary benzylic alcohols. Chem. Commun. (Camb.) 2130–2132 (2009)
Pronin, S. V. & Shenvi, R. A. Synthesis of highly strained terpenes by nonstop tail-to-head polycyclization. Nat. Chem. 4, 915–920 (2012)
Pronin, S. V. & Shenvi, R. A. Synthesis of a potent antimalarial amphilectene. J. Am. Chem. Soc. 134, 19604–19606 (2012)
Miyaoka, H., Shimomura, M., Kimura, H. & Yamada, Y. Antimalarial activity of kalihinol A and new relative diterpenoids from the Okinawan sponge, Acanthella sp. Tetrahedron 54, 13467–13474 (1998)
White, R. D., Keaney, G. F., Slown, C. D. & Wood, J. L. Total synthesis of (±)-kalihinol C. Org. Lett. 6, 1123–1126 (2004)
Srikrishna, A., Ravi, G. & Subbaiah, D. R. C. V. Enantioselective first total syntheses of 2-(formylamino)trachyopsane and ent-2-(isocyano)trachyopsane via a biomimetic approach. Synlett 32–34 (2009)
Corey, E. J. & Magriotis, P. A. Total synthesis and absolute configuration of 7,20-diisocyanoadociane. J. Am. Chem. Soc. 109, 287–289 (1987)
Castellanos, L., Duque, C., Rodríguez, J. & Jiménez, C. Stereoselective synthesis of (−)-4-epiaxinyssamine. Tetrahedron 63, 1544–1552 (2007)
Kitano, Y. et al. Synthesis and antifouling activity of 3-isocyanotheonellin and its analogues. J. Chem. Soc. Perkin Trans. 1 2251–2255 (2002)
Sasaki, T., Nakanishi, A. & Ohno, M. Synthesis of adamantane derivatives. 56. Reaction of 1-adamantyl chloride with trimethylsilyl pseudohalide. J. Org. Chem. 46, 5445–5447 (1981)
Kitano, Y., Chiba, K. & Tada, M. A direct conversion of alcohols to isocyanides. Tetrahedr. Lett. 39, 1911–1912 (1998)
Masutani, K., Minowa, T., Hagiwara, Y. & Mukaiyama, T. Cyanation of alcohols with diethyl cyanophosphonate and 2,6-dimethyl-1,4-benzoquinone by a new type of oxidation–reduction condensation. Bull. Chem. Soc. Jpn. 79, 1106–1117 (2006)
Richard, J. P., Toteva, M. M. & Amyes, T. L. What is the stabilizing interaction with nucleophilic solvents in the transition state for solvolysis of tertiary derivatives: nucleophilic solvent participation or nucleophilic solvation? Org. Lett. 3, 2225–2228 (2001)
Winstein, S., Clippinger, E., Fainberg, A. H., Heck, R. & Robinson, G. C. Salt effects and ion pairs in solvolysis and related reactions. III. 1. Common ion rate depression and exchange of anions during acetolysis. J. Am. Chem. Soc. 78, 328–335 (1956)
Stevens, P. G. & McNiven, N. L. Halogenation of optically active tertiary carbinols. J. Am. Chem. Soc. 61, 1295–1296 (1939)
Kobayashi, S., Hachiya, I., Araki, M. & Ishitani, H. Scandium trifluoromethanesulfonate (Sc(OTf)3). A novel reusable catalyst in the Diels–Alder reaction. Tetrahedr. Lett. 34, 3755–3758 (1993)
Kobayashi, S., Nagayama, S. & Busujima, T. Lewis acid catalysts stable in water. Correlation between catalytic activity in water and hydrolysis constants and exchange rate constants for substitution of inner-sphere water ligands. J. Am. Chem. Soc. 120, 8287–8288 (1998)
Nishikawa, K. et al. Total synthesis of 10-isocyano-4-cadinene and determination of its absolute configuration. Org. Lett. 12, 904–907 (2010)
Kochi, T. & Ellman, J. A. Asymmetric α-alkylation of N′-tert-butanesulfinyl amidines. Application to the total synthesis of (6R,7S)-7-amino-7,8-dihydro-α-bisabolene. J. Am. Chem. Soc. 126, 15652–15653 (2004)
Winstein, S. & Holness, N. J. Neighboring carbon and hydrogen. XIX. t-Butylcyclohexyl derivatives. Quantitative conformational analysis. J. Am. Chem. Soc. 77, 5562–5578 (1955)
Shiner, V. J., Jr & Jewett, J. G. The transition state in acetolysis of cyclohexyl tosylate. J. Am. Chem. Soc. 87, 1384–1385 (1965)
Rauk, A., Sorensen, T. S. & Schleyer, P. V. Tertiary cyclohexyl cations. Definitive evidence for the existence of isomeric structures (hyperconjomers). J. Chem. Soc., Perkin Trans. 2 6, 869–874 (2001)
Ferraris, D., Cox, C., Anand, R. & Lectka, T. C–F bond activation by aryl carbocations: conclusive intramolecular fluoride shifts between carbon atoms in solution and the first examples of intermolecular fluoride ion abstractions. J. Am. Chem. Soc. 119, 4319–4320 (1997)
Lanza, G., Fragalà, I. L. & Marks, T. J. Ligand substituent, anion, and solvation effects on ion pair structure, thermodynamic stability, and structural mobility in ‘constrained geometry’ olefin polymerization catalysts: an ab initio quantum chemical investigation. J. Am. Chem. Soc. 122, 12764–12777 (2000)
Acknowledgements
We thank C. Moore and A. L. Rheingold for X-ray crystallographic analysis. We thank the Yu laboratory for the use of their liquid chromatography–mass spectrometry equipment, the Boger laboratory for the use of their polarimeter, the Baran laboratory for the use of their cold room. Financial support for this work was provided by Eli Lilly, Boehringer Ingelheim and the Scripps Research Institute.
Author information
Authors and Affiliations
Contributions
S.V.P. and R.A.S. conceived the work. S.V.P. and C.A.R. performed the experiments. S.V.P., C.A.R. and R.A.S. designed the experiments and analysed the data. R.A.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Supplementary Data – see contents page for details. This file was replaced on 13 November 2013. (PDF 14777 kb)
Rights and permissions
About this article
Cite this article
Pronin, S., Reiher, C. & Shenvi, R. Stereoinversion of tertiary alcohols to tertiary-alkyl isonitriles and amines. Nature 501, 195–199 (2013). https://doi.org/10.1038/nature12472
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12472
This article is cited by
-
Photoredox-catalyzed C–C bond cleavage of cyclopropanes for the formation of C(sp3)–heteroatom bonds
Nature Communications (2022)
-
Intramolecular substitutions of secondary and tertiary alcohols with chirality transfer by an iron(III) catalyst
Nature Communications (2019)
-
Innovation in protecting-group-free natural product synthesis
Nature Reviews Chemistry (2019)
-
Enantioselective decarboxylative chlorination of β-ketocarboxylic acids
Nature Communications (2017)
-
Metal-catalysed azidation of tertiary C–H bonds suitable for late-stage functionalization
Nature (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.