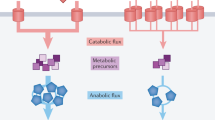
Abstract
The cyclic AMP (cAMP)-dependent catabolite repression effect in Escherichia coli is among the most intensely studied regulatory processes in biology. However, the physiological function(s) of cAMP signalling and its molecular triggers remain elusive. Here we use a quantitative physiological approach to show that cAMP signalling tightly coordinates the expression of catabolic proteins with biosynthetic and ribosomal proteins, in accordance with the cellular metabolic needs during exponential growth. The expression of carbon catabolic genes increased linearly with decreasing growth rates upon limitation of carbon influx, but decreased linearly with decreasing growth rate upon limitation of nitrogen or sulphur influx. In contrast, the expression of biosynthetic genes showed the opposite linear growth-rate dependence as the catabolic genes. A coarse-grained mathematical model provides a quantitative framework for understanding and predicting gene expression responses to catabolic and anabolic limitations. A scheme of integral feedback control featuring the inhibition of cAMP signalling by metabolic precursors is proposed and validated. These results reveal a key physiological role of cAMP-dependent catabolite repression: to ensure that proteomic resources are spent on distinct metabolic sectors as needed in different nutrient environments. Our findings underscore the power of quantitative physiology in unravelling the underlying functions of complex molecular signalling networks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Laub, M. T. & Goulian, M. Specificity in two-component signal transduction pathways. Annu. Rev. Genet. 41, 121–145 (2007)
Potrykus, K. & Cashel, M. (p)ppGpp: still magical? Annu. Rev. Microbiol. 62, 35–51 (2008)
Hengge, R. Principles of c-di-GMP signalling in bacteria. Nature Rev. Microbiol. 7, 263–273 (2009)
Porter, S. L., Wadhams, G. H. & Armitage, J. P. Signal processing in complex chemotaxis pathways. Nature Rev. Microbiol. 9, 153–165 (2011)
Brent, R. Cell signaling: what is the signal and what information does it carry? FEBS Lett. 583, 4019–4024 (2009)
Purvis, J. E. et al. p53 dynamics control cell fate. Science 336, 1440–1444 (2012)
Hao, N., Budnik, B. A., Gunawardena, J. & O’Shea, E. K. Tunable signal processing through modular control of transcription factor translocation. Science 339, 460–464 (2013)
Young, J. W., Locke, J. C. & Elowitz, M. B. Rate of environmental change determines stress response specificity. Proc. Natl Acad. Sci. USA 110, 4140–4145 (2013)
Makman, R. S. & Sutherland, E. W. Adenosine 3′,5′-phosphate in Escherichia coli. J. Biol. Chem. 240, 1309–1314 (1965)
Perlman, R. L., De Crombrugghe, B. & Pastan, I. Cyclic AMP regulates catabolite and transient repression in E. coli. Nature 223, 810–812 (1969)
Magasanik, B. Catabolite repression. Cold Spring Harb. Symp. Quant. Biol. 26, 249–256 (1961)
Epps, H. M. & Gale, E. F. The influence of the presence of glucose during growth on the enzymic activities of Escherichia coli: comparison of the effect with that produced by fermentation acids. Biochem. J. 36, 619–623 (1942)
Neidhardt, F. C. & Magasanik, B. Effect of mixtures of substrates on the biosynthesis of inducible enzymes in Aerobacter aerogenes. J. Bacteriol. 73, 260–263 (1957)
Kolb, A., Busby, S., Buc, H., Garges, S. & Adhya, S. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62, 749–797 (1993)
Saier, M. H., Jr, Feucht, B. U. & Hofstadter, L. J. Regulation of carbohydrate uptake and adenylate cyclase activity mediated by the enzymes II of the phosphoenolpyruvate: sugar phosphotransferase system in Escherichia coli. J. Biol. Chem. 251, 883–892 (1976)
Deutscher, J., Francke, C. & Postma, P. W. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70, 939–1031 (2006)
Epstein, W., Rothman-Denes, L. B. & Hesse, J. Adenosine 3′:5′-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc. Natl Acad. Sci. USA 72, 2300–2304 (1975)
Hogema, B. M. et al. Catabolite repression by glucose 6-phosphate, gluconate and lactose in Escherichia coli. Mol. Microbiol. 24, 857–867 (1997)
Bettenbrock, K. et al. Correlation between growth rates, EIIACrr phosphorylation, and intracellular cyclic AMP levels in Escherichia coli K-12. J. Bacteriol. 189, 6891–6900 (2007)
Mandelstam, J. The repression of constitutive beta-galactosidase in Escherichia coli by glucose and other carbon sources. Biochem. J. 82, 489–493 (1962)
McFall, E. & Magasanik, B. Effects of thymine and of phosphate deprivation on enzyme synthesis in Escherichia coli. Biochim. Biophys. Acta 55, 900–908 (1962)
Clark, D. J. & Marr, A. G. Studies on the repression of beta-galactosidase in Escherichia coli. Biochim. Biophys. Acta 92, 85–94 (1964)
Ullmann, A., Tillier, F. & Monod, J. Catabolite modulator factor: a possible mediator of catabolite repression in bacteria. Proc. Natl Acad. Sci. USA 73, 3476–3479 (1976)
Narang, A. & Pilyugin, S. S. Bacterial gene regulation in diauxic and non-diauxic growth. J. Theor. Biol. 244, 326–348 (2007)
Monod, J. The phenomenon of enzymatic adaptation - and its bearings on problems of genetics and cellular differentiation. Growth 11, 223–289 (1947)
Okada, T. et al. Role of inducer exclusion in preferential utilization of glucose over melibiose in diauxic growth of Escherichia coli. J. Bacteriol. 146, 1030–1037 (1981)
Inada, T., Kimata, K. & Aiba, H. Mechanism responsible for glucose-lactose diauxie in Escherichia coli: challenge to the cAMP model. Genes Cells 1, 293–301 (1996)
Müller-Hill, B. The lac Operon: a Short History of a Genetic Paradigm. (de Gruyter, 1996)
Kuhlman, T., Zhang, Z., Saier, M. H., Jr & Hwa, T. Combinatorial transcriptional control of the lactose operon of Escherichia coli. Proc. Natl Acad. Sci. USA 104, 6043–6048 (2007)
Wanner, B. L., Kodaira, R. & Neidhardt, F. C. Regulation of lac operon expression: reappraisal of the theory of catabolite repression. J. Bacteriol. 136, 947–954 (1978)
Kuo, J. T., Chang, Y. J. & Tseng, C. P. Growth rate regulation of lac operon expression in Escherichia coli is cyclic AMP dependent. FEBS Lett. 553, 397–402 (2003)
Klumpp, S., Zhang, Z. & Hwa, T. Growth rate-dependent global effects on gene expression in bacteria. Cell 139, 1366–1375 (2009)
Scott, M., Gunderson, C. W., Mateescu, E. M., Zhang, Z. & Hwa, T. Interdependence of cell growth and gene expression: origins and consequences. Science 330, 1099–1102 (2010)
Reitzer, L. Nitrogen assimilation and global regulation in Escherichia coli. Annu. Rev. Microbiol. 57, 155–176 (2003)
Kim, M. et al. Need-based activation of ammonium uptake in Escherichia coli. Mol. Syst. Biol. 8, 616 (2012)
Silverstone, A. E., Arditti, R. R. & Magasanik, B. Catabolite-insensitive revertants of lac promoter mutants. Proc. Natl Acad. Sci. USA 66, 773–779 (1970)
Schaechter, M., Maaloe, O. & Kjeldgaard, N. O. Dependency on medium and temperature of cell size and chemical composition during balanced growth of Salmonella typhimurium. J. Gen. Microbiol. 19, 592–606 (1958)
Maaloe, O. in Biological Regulation and Development 487–542 (Plenum, 1979)
Bender, R. A. & Magasanik, B. Regulatory mutations in the Klebsiella aerogenes structural gene for glutamine synthetase. J. Bacteriol. 132, 100–105 (1977)
Scott, M. & Hwa, T. Bacterial growth laws and their applications. Curr. Opin. Biotechnol. 22, 559–565 (2011)
Schuetz, R., Zamboni, N., Zampieri, M., Heinemann, M. & Sauer, U. Multidimensional optimality of microbial metabolism. Science 336, 601–604 (2012)
Goyal, S., Yuan, J., Chen, T., Rabinowitz, J. D. & Wingreen, N. S. Achieving optimal growth through product feedback inhibition in metabolism. PLOS Comput. Biol. 6, e1000802 (2010)
Doucette, C. D., Schwab, D. J., Wingreen, N. S. & Rabinowitz, J. D. α-ketoglutarate coordinates carbon and nitrogen utilization via enzyme I inhibition. Nature Chem. Biol. 7, 894–901 (2011)
Yan, D., Lenz, P. & Hwa, T. Overcoming fluctuation and leakage problems in the quantification of intracellular 2-oxoglutarate levels in Escherichia coli. Appl. Environ. Microbiol. 77, 6763–6771 (2011)
Leigh, J. R. Control Theory (The Institution of Electrical Engineers, 2004)
Peterkofsky, A. & Gazdar, C. Escherichia coli adenylate cyclase complex: regulation by the proton electrochemical gradient. Proc. Natl Acad. Sci. USA 76, 1099–1103 (1979)
Tyler, B. & Magasanik, B. Physiological basis of transient repression of catabolic enzymes in Escherichia coli. J. Bacteriol. 102, 411–422 (1970)
Harwood, J. P. & Peterkofsky, A. Glucose-sensitive adenylate cyclase in toluene-treated cells of Escherichia coli B. J. Biol. Chem. 250, 4656–4662 (1975)
Acknowledgements
We are grateful to R. Bender, A. Danchin, P. Geiduschek, J. Ingraham, S. Kustu, W. F. Loomis, A. Narang, J. Rabinowitz, M. H. Saier and members of the Hwa laboratory for valuable comments. This work was supported by the Human Frontiers in Science Program (RGP0022), and by the NSF to T.H. (PHY1058793) and through the Center for Theoretical Biological Physics (PHY0822283).
Author information
Authors and Affiliations
Contributions
C.Y., D.Y. and T.H. designed the study. C.Y., H.O., S.H., Z.Z. M.K., C.W.G. and D.Y. performed experiments. C.Y., S.H., Y.P.W. and T.H. analysed the data. P.L. and T.H. developed the model. All authors contributed to writing the paper and the supplement.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Materials and Methods, Supplementary Notes, Supplementary Tables 1-19, Supplementary Figures 1-36 and Supplementary References. (PDF 6595 kb)
Rights and permissions
About this article
Cite this article
You, C., Okano, H., Hui, S. et al. Coordination of bacterial proteome with metabolism by cyclic AMP signalling. Nature 500, 301–306 (2013). https://doi.org/10.1038/nature12446
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12446
This article is cited by
-
Inherited chitinases enable sustained growth and rapid dispersal of bacteria from chitin particles
Nature Microbiology (2023)
-
Shaping bacterial gene expression by physiological and proteome allocation constraints
Nature Reviews Microbiology (2023)
-
Inference of transcriptome signatures of Escherichia coli in long-term stationary phase
Scientific Reports (2023)
-
Functional decomposition of metabolism allows a system-level quantification of fluxes and protein allocation towards specific metabolic functions
Nature Communications (2023)
-
Unbalanced response to growth variations reshapes the cell fate decision landscape
Nature Chemical Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.