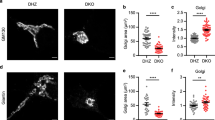
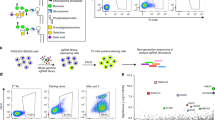
Abstract
Newly synthesized proteins and lipids are transported across the Golgi complex via different mechanisms whose respective roles are not completely clear. We previously identified a non-vesicular intra-Golgi transport pathway for glucosylceramide (GlcCer)—the common precursor of the different series of glycosphingolipids—that is operated by the cytosolic GlcCer-transfer protein FAPP2 (also known as PLEKHA8) (ref. 1). However, the molecular determinants of the FAPP2-mediated transfer of GlcCer from the cis-Golgi to the trans-Golgi network, as well as the physiological relevance of maintaining two parallel transport pathways of GlcCer—vesicular and non-vesicular—through the Golgi, remain poorly defined. Here, using mouse and cell models, we clarify the molecular mechanisms underlying the intra-Golgi vectorial transfer of GlcCer by FAPP2 and show that GlcCer is channelled by vesicular and non-vesicular transport to two topologically distinct glycosylation tracks in the Golgi cisternae and the trans-Golgi network, respectively. Our results indicate that the transport modality across the Golgi complex is a key determinant for the glycosylation pattern of a cargo and establish a new paradigm for the branching of the glycosphingolipid synthetic pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
D’Angelo, G. et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature 449, 62–67 (2007)
Hakomori, S. I. Structure and function of glycosphingolipids and sphingolipids: recollections and future trends. Biochim. Biophys. Acta 1780, 325–346 (2008)
Jeckel, D., Karrenbauer, A., Burger, K. N., van Meer, G. & Wieland, F. Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions. J. Cell Biol. 117, 259–267 (1992)
Futerman, A. H. & Pagano, R. E. Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem. J. 280, 295–302 (1991)
Hannun, Y. A. & Obeid, L. M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature Rev. Mol. Cell Biol. 9, 139–150 (2008)
Halter, D. et al. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J. Cell Biol. 179, 101–115 (2007)
Maccioni, H. J., Quiroga, R. & Ferrari, M. L. Cellular and molecular biology of glycosphingolipid glycosylation. J. Neurochem. 117, 589–602 (2011)
Jacewicz, M., Clausen, H., Nudelman, E., Donohue-Rolfe, A. & Keusch, G. T. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J. Exp. Med. 163, 1391–1404 (1986)
van Heyningen, S. V. Cholera toxin: interaction of subunits with ganglioside GM1 . Science 183, 656–657 (1974)
Psotka, M. A. et al. Shiga toxin 2 targets the murine renal collecting duct epithelium. Infect. Immun. 77, 959–969 (2009)
Okuda, T. et al. Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. J. Biol. Chem. 281, 10230–10235 (2006)
D’Angelo, G., Vicinanza, M. & De Matteis, M. A. Lipid-transfer proteins in biosynthetic pathways. Curr. Opin. Cell Biol. 20, 360–370 (2008)
Chege, N. W. & Pfeffer, S. R. Compartmentation of the Golgi complex: brefeldin-A distinguishes trans-Golgi cisternae from the trans-Golgi network. J. Cell Biol. 111, 893–899 (1990)
Godi, A. et al. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nature Cell Biol. 6, 393–404 (2004)
Yamaji, T., Nishikawa, K. & Hanada, K. Transmembrane BAX inhibitor motif containing (TMBIM) family proteins perturbs a trans-Golgi network enzyme, Gb3 synthase, and reduces Gb3 biosynthesis. J. Biol. Chem. 285, 35505–35518 (2010)
San Pietro, E. et al. Group IV phospholipase A2α controls the formation of inter-cisternal continuities involved in intra-Golgi transport. PLoS Biol. 7, e1000194 (2009)
Ichikawa, S., Nakajo, N., Sakiyama, H. & Hirabayashi, Y. A mouse B16 melanoma mutant deficient in glycolipids. Proc. Natl Acad. Sci. USA 91, 2703–2707 (1994)
He, J. et al. Molecular basis of phosphatidylinositol 4-phosphate and ARF1 GTPase recognition by the FAPP1 pleckstrin homology (PH) domain. J. Biol. Chem. 286, 18650–18657 (2011)
D’Angelo, G., Vicinanza, M., Di Campli, A. & De Matteis, M. A. The multiple roles of PtdIns(4)P–not just the precursor of PtdIns(4,5)P2. J. Cell Sci. 121, 1955–1963 (2008)
Kamlekar, R. K. et al. The glycolipid transfer protein (GLTP) domain of phosphoinositol 4-phosphate adaptor protein-2 (FAPP2): structure drives preference for simple neutral glycosphingolipids. Biochim. Biophys. Acta 1831, 417–427 (2013)
Yan, X., Watson, J., Ho, P. S. & Deinzer, M. L. Mass spectrometric approaches using electrospray ionization charge states and hydrogen-deuterium exchange for determining protein structures and their conformational changes. Mol. Cell. Proteomics 3, 10–23 (2004)
Sato, T. et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature 448, 366–369 (2007)
D’Angelo, G. et al. GRASP65 and GRASP55 sequentially promote the transport of C-terminal valine-bearing cargos to and through the Golgi complex. J. Biol. Chem. 284, 34849–34860 (2009)
Zhai, X. et al. Glycolipid acquisition by human glycolipid transfer protein dramatically alters intrinsic tryptophan fluorescence: insights into glycolipid binding affinity. J. Biol. Chem. 284, 13620–13628 (2009)
Ohvo-Rekilä, H. & Mattjus, P. Monitoring glycolipid transfer protein activity and membrane interaction with the surface plasmon resonance technique. Biochim. Biophys. Acta 1808, 47–54 (2011)
Burke, J. E., Perisic, O., Masson, G. R., Vadas, O. & Williams, R. L. Oncogenic mutations mimic and enhance dynamic events in the natural activation of phosphoinositide 3-kinase p110alpha (PIK3CA). Proc. Natl Acad. Sci. USA 109, 15259–15264 (2012)
Mallard, F. & Johannes, L. Shiga toxin B-subunit as a tool to study retrograde transport. Methods Mol. Med. 73, 209–220 (2003)
Muramatsu, K. et al. Neuron-specific recombination by Cre recombinase inserted into the murine tau locus. Biochem. Biophys. Res. Commun. 370, 419–423 (2008)
Hashimoto, Y. et al. Neuron-specific and inducible recombination by Cre recombinase in the mouse. Neuroreport 19, 621–624 (2008)
Bolte, S. & Cordelieres, F. P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006)
Acknowledgements
We thank A. Luini, C. Wilson and D. Priestman for discussions, A. Egorova for help with electron microscopy, G. Liebisch, A. Sigruener and G. Schmitz for lipidomic analysis. M.A.D.M. acknowledges the support of Telethon (GSP08002 and GGP06166), Associazione Italiana per la Ricerca sul Cancro (AIRC) (IG 8623), and the EU (FP7 Lipidomicnet). G.D.’A. acknowledges the support of AIRC (MFAG 10585). P.M. acknowledges the support of Academy of Finland and Sigrid Jusélius Foundation. C.-C.C. was funded by a Study Abroad Scholarship from the Taiwan Ministry of Education.
Author information
Authors and Affiliations
Contributions
M.A.D.M. supervised the entire project; M.A.D.M. and G.D.’A. wrote the manuscript with comments from all co-authors; G.D.’A., with the help of M.S., designed and conducted at TIGEM the experiments of sphingolipid labelling, membrane trafficking, immuno-localization and controlled proteolysis. M.S. designed the strategy and produced plasmid vectors. M.S. and G.D.T. prepared recombinant proteins, anti-FAPP2 and anti-BET3 antibodies. T.U. and T.S. generated and characterized FAPP2geo/geo and FAPP2−/−mice under the supervision of A.H. C.-C.C. conducted the HPLC measurements of GSLs under the supervision of F.M.P. L.J. provided the Cy3-ShTxB. E.P. and T.D. conducted the electron microscopy experiments. H.O.-R. conducted the surface plasmon resonance experiments under the supervision of P.M. A.V. conducted the tryptophan fluorescence and circular dichroism experiments under the supervision of S.D’.A. F.C. and G.D’.A. produced the mathematical model for GSL metabolism. M.M. and P.P. performed and interpreted the MS analysis; R.L.W. and J.E.B. performed and interpreted the HDX analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Data, Supplementary References, Supplementary Figures 1-13 and Supplementary Tables 1-2. (PDF 31231 kb)
Rights and permissions
About this article
Cite this article
D’Angelo, G., Uemura, T., Chuang, CC. et al. Vesicular and non-vesicular transport feed distinct glycosylation pathways in the Golgi. Nature 501, 116–120 (2013). https://doi.org/10.1038/nature12423
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12423
This article is cited by
-
It started with a western
Nature Cell Biology (2024)
-
Glycosphingolipid metabolism and its role in ageing and Parkinson’s disease
Glycoconjugate Journal (2022)
-
Cross-talks of glycosylphosphatidylinositol biosynthesis with glycosphingolipid biosynthesis and ER-associated degradation
Nature Communications (2020)
-
Sphingolipid metabolism in cancer signalling and therapy
Nature Reviews Cancer (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.