Abstract
Avian influenza A viruses rarely infect humans; however, when human infection and subsequent human-to-human transmission occurs, worldwide outbreaks (pandemics) can result. The recent sporadic infections of humans in China with a previously unrecognized avian influenza A virus of the H7N9 subtype (A(H7N9)) have caused concern owing to the appreciable case fatality rate associated with these infections (more than 25%), potential instances of human-to-human transmission1, and the lack of pre-existing immunity among humans to viruses of this subtype. Here we characterize two early human A(H7N9) isolates, A/Anhui/1/2013 (H7N9) and A/Shanghai/1/2013 (H7N9); hereafter referred to as Anhui/1 and Shanghai/1, respectively. In mice, Anhui/1 and Shanghai/1 were more pathogenic than a control avian H7N9 virus (A/duck/Gunma/466/2011 (H7N9); Dk/GM466) and a representative pandemic 2009 H1N1 virus (A/California/4/2009 (H1N1pdm09); CA04). Anhui/1, Shanghai/1 and Dk/GM466 replicated well in the nasal turbinates of ferrets. In nonhuman primates, Anhui/1 and Dk/GM466 replicated efficiently in the upper and lower respiratory tracts, whereas the replicative ability of conventional human influenza viruses is typically restricted to the upper respiratory tract of infected primates. By contrast, Anhui/1 did not replicate well in miniature pigs after intranasal inoculation. Critically, Anhui/1 transmitted through respiratory droplets in one of three pairs of ferrets. Glycan arrays showed that Anhui/1, Shanghai/1 and A/Hangzhou/1/2013 (H7N9) (a third human A(H7N9) virus tested in this assay) bind to human virus-type receptors, a property that may be critical for virus transmissibility in ferrets. Anhui/1 was found to be less sensitive in mice to neuraminidase inhibitors than a pandemic H1N1 2009 virus, although both viruses were equally susceptible to an experimental antiviral polymerase inhibitor. The robust replicative ability in mice, ferrets and nonhuman primates and the limited transmissibility in ferrets of Anhui/1 suggest that A(H7N9) viruses have pandemic potential.
Similar content being viewed by others
Main
Influenza A virus infections place a considerable burden on public health and the world economy. In March 2013, several individuals were reported to be infected with an avian A(H7N9) virus1,2. Viruses of this subtype do not circulate in humans, so A(H7N9) viruses capable of transmitting among humans would encounter populations that lack any protective immunity to them. By May 30 2013, 132 confirmed human infections with A(H7N9) viruses had been reported, with 37 deaths3, resulting in a case fatality rate of >25%.
Sequence and phylogenetic analysis revealed that the haemagglutinin (HA) and neuraminidase (NA) genes of the A(H7N9) viruses originated from avian H7 and N9 viruses, respectively2,4,5, whereas the remaining six genes are closely related to H9N2 subtype viruses that have circulated in poultry in China2,4,5. Several of the A(H7N9) viruses possess amino acid changes known to facilitate infection of mammals, such as leucine at position 226 of HA (H3 HA numbering), which confers increased binding to human-type receptors6, and the mammalian-adapting mutations E627K7,8 or D701N9 in the PB2 polymerase subunit. Notably, the PB2-627K or PB2-701N markers have been detected in almost all human, but not avian or environmental, A(H7N9) isolates, suggesting ready adaptation of A(H7N9) viruses to humans.
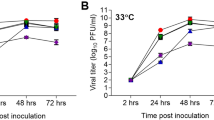
To characterize the biological properties and pandemic potential of A(H7N9) viruses, we compared Anhui/1 (which possesses the mammalian-adapting HA-226L and PB2-627K markers) and Shanghai/1 (which possesses the ‘avian-type’ HA-226Q and mammalian-adapting PB2-627K markers) with the phylogenetically unrelated avian H7N9 Dk/GM466 virus, and with CA04, an early, representative 2009 H1N1 pandemic virus. Anhui/1, Shanghai/1 and CA04 replicated efficiently in Madin–Darby canine kidney (MDCK) cells and in differentiated normal human bronchial epithelial cells compared with Dk/GM466, especially at 33 °C, a temperature corresponding to the human upper airway (Supplementary Fig. 1). Electron microscopic analysis showed Anhui/1 as a spherical particle that appeared to be efficiently released from infected cells (Supplementary Fig. 2).
Next, we assessed the pathogenicity of Anhui/1 and Shanghai/1 in established animal models in influenza virus research, namely mice, ferrets and nonhuman primates (Anhui/1 only). In BALB/c mice, Anhui/1 and Shanghai/1 were more pathogenic than CA04 and Dk/GM466 on the basis of MLD50 (mouse lethal dose 50; the dose required to kill 50% of infected mice) values, which were 103.5 plaque-forming units (p.f.u.) for Anhui/1 and Shanghai/1, 105.5 p.f.u. for CA04 and 106.7 p.f.u. for Dk/GM466 (Supplementary Fig. 3). Three days post-infection (d.p.i.), virus titres in the lungs and nasal turbinates of Anhui/1-, Shanghai/1- and CA04-infected mice were slightly higher than those in Dk/GM466-infected mice (Supplementary Table 1). Lung lesions in Anhui/1- and CA04-infected mice were more severe than those in Dk/GM466-infected mice, in particular at 6 d.p.i. (Supplementary Fig. 4). Bronchitis, bronchiolitis, thickening of the alveolar septa, oedema and interstitial inflammatory cell infiltration were also more prominent in Anhui/1- and CA04-infected mice. Viral antigen was detected in many alveolar and bronchial epithelial cells at 3 d.p.i. in Anhui/1- and CA04-infected mice (Supplementary Fig. 4), whereas viral-antigen-positive cells were restricted to a few bronchial epithelial cells in Dk/GM466-infected mice (Supplementary Fig. 4). Collectively, these findings demonstrate that Anhui/1 is as pathogenic as CA04 and more pathogenic than Dk/GM466 in mice.
Ferrets intranasally infected with Anhui/1, Shanghai/1, CA04 or Dk/GM466 experienced loss of appetite. Transient weight loss was detected in one of the three animals infected with Anhui/1 (Supplementary Fig. 5). Virus titres in the trachea of Anhui/1-, Shanghai/1- and CA04-infected ferrets were higher at 3 d.p.i. than those obtained from Dk/GM466-infected animals (Supplementary Table 2); at 6 d.p.i., virus was isolated from the trachea of Anhui/1-, Shanghai/1- and Dk/GM466-infected animals, but not from that of CA04-infected ferrets. All three viruses replicated inefficiently in the lungs of these animals. In this study, we did not detect virus in the lungs in the CA04-infected animals at 3 d.p.i. (Supplementary Table 2), whereas this virus was recovered from two out of three ferrets infected at 3 d.p.i. in our previous study10, consistent with efficient replication of pandemic 2009 H1N1 virus in the lungs of ferrets as reported by others11,12,13,14. Appreciable amounts of virus were recovered from nasal turbinates at 3 and 6 d.p.i., with the exception of the nasal turbinates of CA04-infected animals at 6 d.p.i. (Supplementary Table 2). Inflammation was prominent in the trachea and submucosal glands of CA04-infected ferrets (Supplementary Fig. 6). Viral-antigen-positive cells were detected in the tracheal, glandular and alveolar epithelial cells of all ferrets. Anhui/1- and CA04-infected ferrets displayed numerous viral-antigen-positive cells, especially in the glandular epithelia, whereas Dk/GM466-infected ferrets presented far fewer antigen-positive cells. Viral antigens were also detected in pneumocytes in localized lung lesions of each ferret. In mediastinal lymph nodes, viral antigen was detected in Anhui/1- and CA04-infected ferrets only. Anhui/1 and Shanghai/1 thus established a robust, although relatively mild, infection in the upper respiratory organs of ferrets that was unlike most avian H5N1 influenza virus infections in ferrets, which consistently cause severe symptoms including profound weight loss.
Infection of cynomolgus macaques (Macaca fascicularis) with 6.7 × 107 p.f.u. of Anhui/1 or Dk/GM466 caused fever (Supplementary Fig. 7), as did infection with CA04 in our previous study10. Both Anhui/1 and Dk/GM466 replicated appreciably in macaque nasal turbinates, trachea and lungs, although variability among the virus titres was noticed, as is commonly found among outbred animals (Supplementary Tables 3 and 4a). Previously, we detected efficient replication of CA04 in the respiratory organs of cynomolgus macaques at 3 d.p.i.; by 7 d.p.i., no virus was detected in the lungs10.
Pathological examination of the respiratory organs did not reveal major differences between macaques infected with Anhui/1 or Dk/GM466; no notable lesions were detected in the trachea or lobular bronchus, but alveolar spaces contained oedematous exudate and inflammatory infiltrates comprising mainly neutrophils and monocytes/macrophages (Fig. 1). At 6 d.p.i., regenerative changes were observed. Lung inflammation scores did not reveal appreciable differences between animals infected with Anhui/1 or Dk/GM466 (Supplementary Table 4b). Numerous tracheal and bronchial epithelial cells of Anhui/1-infected macaques were positive for viral antigen (Fig. 1b, d, f, h), as was observed in our previous study with CA04 at 7 d.p.i.10. Fewer antigen-positive cells in the tracheal and bronchial epithelia of Dk/GM466-infected animals were detected, in particular at 3 d.p.i. (Fig. 1n, r). Viral antigen was also detected in the mediastinal lymph node sections of Anhui/1-infected monkeys (Supplementary Fig. 8). In addition, our analysis of chemokine/cytokine responses suggests that Anhui/1 induces strong inflammatory responses both systemically and at the site of virus infection (Supplementary Fig. 9 and Supplementary Information).
a–x, Shown are pathological findings in the trachea (a–d, m–p), bronchus (e–h, q–t) and lungs (i–l, u–x) of macaques infected with Anhui/1 (a–l) or Dk/GM466 (m–x) at 3 d.p.i. (a, b, e, f, i, j, m, n, q, r, u, v) or 6 d.p.i. (c, d, g, h, k, l, o, p, s, t, w, x), with haematoxylin and eosin staining (a, c, e, g, i, k, m, o, q, s, u, w) or immunohistochemistry for influenza viral antigen (b, d, f, h, j, l, n, p, r, t, v, x). y, Haematoxylin and eosin staining of the lung of an uninfected macaque is shown. Original magnifications: ×400 (a–h, m–t), ×200 (i–l, u–y).
No sustained human-to-human transmission of the novel A(H7N9) viruses has been reported to date. To assess the transmissibility of Anhui/1, naive ‘contact’ ferrets in wireframe cages (which prevent direct contact with animals in neighbouring cages, but allow respiratory droplet transmission) were placed adjacent to an ‘infected’ ferret the day after infection as described previously15. We recovered viruses from the nasal washes of all three contact ferrets for CA04 (Fig. 2), as expected on the basis of previous studies by us10 and others13,14. No virus was detected in the nasal washes of contact ferrets for Dk/GM466 (Fig. 2), consistent with the general lack of transmissibility of avian influenza viruses in ferrets. However, one of three contact ferrets for Anhui/1 shed virus on days 3–7 after contact (Fig. 2). Serum antibody titres against Anhui/1 confirmed infection of this animal, whereas the other two contact animals did not seroconvert (Supplementary Table 5). Given that avian H5N1 influenza viruses require several mutations to transmit through respiratory droplets among ferrets15,16,17,18, the pandemic potential of A(H7N9) viruses may be greater than that of the highly pathogenic avian H5N1 influenza viruses.
Ferrets were infected with 5 × 105 p.f.u. of Anhui/1, Dk/GM466 or CA04 (infected ferrets). One day later, three naive ferrets (contact ferrets) were each placed in a cage adjacent to an infected ferret. Nasal washes were collected from infected ferrets on day 1 after inoculation and from contact ferrets on day 1 after co-housing, and then every other day (up to 9 days) for virus titration.
Because replication and transmission of an H5 HA-possessing virus in ferrets is associated with amino acid changes in HA15,18, we sequenced the genomes of viruses obtained from infected and contact ferrets. Compared to virus inoculum, we detected three non-synonymous mutations in HA (T71I, R131K and A135T; Fig. 3 and Supplementary Table 6) and one nonsynonymous mutation in NA (A27T; N9 numbering) (Supplementary Table 6); we also detected several synonymous nucleotide changes. The amino acid mutations in HA were detected in >40% of the molecular clones derived from samples obtained on day 3 post-contact (Supplementary Table 7), but were found exclusively by day 5 post-contact. Interestingly, the egg-grown virus stock of Anhui/1 possessed a mixture of amino acids at five positions, and the three amino acid changes in HA detected in the transmitted virus were detected in two of 39 HA clones representing the egg-grown virus stock of Anhui/1 (Supplementary Table 7). The selection of these mutations during A/Anhui replication and/or transmission in ferrets strongly suggests that these mutations have a biological role, possibly in HA stability and/or receptor-binding specificity or affinity. In fact, positions 131 and 135 are located near the receptor-binding pocket (Fig. 3). At position 71, both threonine and isoleucine are commonly found among H7 HAs, and the location of this position ‘underneath’ the receptor-binding pocket suggests a possible effect on HA stability (Fig. 3).
a, Localization of amino acid changes in a virus from a ferret infected via respiratory droplets. Shown is the three-dimensional structure of A/Netherlands/219/2003 (H7N7) HA (Protein data bank (PDB) code, 4DJ6) in complex with human receptor analogues. b, Close-up view of the globular head. Mutations that increase affinity to human-type receptors are shown in cyan. Mutations that emerged in Anhui/1 HA during replication and/or transmission in ferrets are shown in red (T71I), green (A135T) and blue (R131K). The human receptor analogue (derived from its complex with H9 HA (PDB, 1JSI); shown in orange) is docked into the structure. Images were created with MacPymol (http://www.pymol.org/). c–g, Receptor specificities of recombinant viruses possessing A(H7N9) HAs (Anhui/1, Shanghai/1, Hangzhou/1) were compared with representative avian (A/Vietnam/1203/2004 (H5N1); Vietnam/1203) and human (A/Kawasaki/173/2001 (H1N1); Kawasaki/173) isolates in a glycan microarray containing α2,3 and α2,6 sialosides. Error bars represent standard deviations calculated from six replicate spots of each glycan. A complete list of glycans is found in Supplementary Table 9.
Our data indicate that Anhui/1 efficiently infects mammalian cells. We speculated that amino acid changes in Anhui/1 HA contribute to this host tropism. The HA-226L residue found in Anhui/1 is known to increase the affinity of H3 HAs (which are phylogenetically closely related to H7 HAs) to sialic acids linked to galactose by an α2,6-linkage (‘human-type’ receptors)6; by contrast, avian influenza viruses preferentially bind to sialic acids linked to galactose by an α2,3-linkage (‘avian-type’ receptors). In addition, Anhui/1 HA deviates from the avian virus consensus sequence at positions 186 and 189, which influence the receptor-binding preferences of H5 and H9 HAs, respectively19,20,21.
To analyse receptor-binding preference, we subjected recombinant viruses possessing the Anhui/1, Shanghai/1 or Hangzhou/1 HA genes (see Supplementary Table 8) in combination with Anhui/1 NA genes and the remaining genes from A/Puerto Rico/8/34 (H1N1) (a laboratory-adapted strain) to glycan array analysis. All three viruses bound to α2,6-linked sialosides, unlike a representative avian virus (A/Vietnam/1203/2004 (H5N1); Vietnam/1203), which showed typical specificity for α2,3-linked sialosides (Fig. 3 and Supplementary Table 9). Anhui/1 and Hangzhou/1 HAs bound most strongly to α2,6-linked sialosides, and in particular to extended N-linked glycans that are found on human bronchial epithelial cells22 (Fig. 3). This binding pattern may be influenced by the ‘human-type’ residues at position 226 (Anhui/1 possesses HA-226L and Hangzhou/1 possesses HA-226I, the residues also found in human H3N2 influenza viruses at this position). Shanghai/1 HA (encoding the ‘avian-type’ HA-226Q) was less selective, binding equally well to both α2,6- and α2,3-linked sialosides (Fig. 3). The specificity of the recombinant Anhui/1 and Hangzhou/1 HA viruses was comparable to the ‘human-type’ receptor specificity of H5N1 virus receptor mutants that exhibit respiratory droplet transmission in ferrets15,23, and to that of the human H7N3 A/New York/107/2003 isolate that exhibits contact, but not respiratory droplet transmission, in ferrets24. Notably, however, inhibition of the NA enzymatic function by inclusion of the neuraminidase inhibitor zanamivir in the glycan array analysis resulted in substantial increases in binding to α2,3-linked sialosides (as shown for Hangzhou/1 in Supplementary Fig. 10). Moreover, preliminary analysis of the specificity of the recombinant Anhui/1 H7 HA revealed preferential binding to α2,3 sialosides (R.P.d.V., R.M. & J.C.P., unpublished observations). Thus, the ‘human-type’ receptor specificity of A(H7N9) viruses assessed by glycan array seems to reflect the combined activities of HA and NA.
In pigs, we observed a mild infection with no clinical symptoms (Supplementary Tables 10 and 11, Supplementary Fig. 11 and Supplementary Information). Signs of disease were also absent in infected chickens and quails, which supported virus replication in a limited number of organs (a characteristic of low pathogenic avian influenza viruses) (Supplementary Tables 12–15 and Supplementary Information).
Antiviral compounds are currently the only therapeutic and prophylactic option for A(H7N9) infections. We therefore determined the in vitro 50% inhibitory concentration (IC50) of several NA inhibitors (oseltamivir, zanamivir, laninamivir and peramivir), and of an experimental inhibitor of the viral RNA polymerase (favipiravir, also known as T-705) against egg-grown virus stocks of Anhui/1 and Shanghai/1. Sanger sequencing of the Shanghai/1 NA gene revealed the R294K mutation known to confer resistance to NA inhibitors for N2 and N9 NAs25. Both Anhui/1 and Shanghai/1 were sensitive to all NA inhibitors tested (Supplementary Table 16), consistent with a recent report that Shanghai/1 is susceptible to oseltamivir and zanamivir26, but inconsistent with the presence of the R294K mutation. A possible explanation for this discrepancy is that the Shanghai/1 isolate contained a mixture of drug-sensitive and -resistant NA genes in which the drug-resistant subpopulation may not have been detected, as described in ref. 27. In fact, plaque purification of the egg-grown Shanghai/1 virus stock revealed a mixed population of NA genes encoding NA-294R or -294K. Testing of these variants confirmed that Shanghai/1-NA-294R was susceptible to NA inhibitors, whereas the NA-294K variant was not (Supplementary Table 16). Further testing demonstrated that 30% of oseltamivir-sensitive virus in the mixture is sufficient for results to be consistent with a purely sensitive virus population (Supplementary Table 17). The IC50 values of favipiravir, determined by plaque-reduction assays in MDCK cells, were low for Anhui/1 and the control CA04 virus (1.4 μg ml−1 and 1.2 μg ml−1, respectively), suggesting that this compound could be a treatment option against A(H7N9) viruses resistant to NA inhibitors.
We also evaluated the therapeutic efficacy of the anti-influenza drugs in mice infected with Anhui/1, CA04 or a recombinant virus possessing the Shanghai/1 NA gene (encoding NA-294K) with the remaining genes from Anhui/1. Peramivir, which is structurally similar to oseltamivir but is administered intravenously, was omitted from these experiments. Mice infected with 103 and 104 p.f.u. of viruses were treated with the drugs beginning at 2 h post-infection. Some NA inhibitors had modest effects on the body-weight loss of Anhui/1-infected mice (Fig. 4a–c), consistent with limited, although statistically significant, effects on virus titre reduction (Fig. 4d–f). We currently do not know whether neuraminidase-resistant variants arose during Anhui/1 replication in mice, which may have limited virus susceptibility to NA inhibitors. In this context, it is interesting to note the poor efficacy of oseltamivir when used to treat a person infected with A(H7N9)28, presumably owing to the emergence of drug-resistant variants. By contrast, favipiravir, which targets the viral polymerase complex, showed clear therapeutic effectiveness against both viruses at both doses tested.
a–f, Mice were intranasally inoculated with 103 or 104 p.f.u. (50 μl) of Anhui/1 (a, d), CA04 (b, e) or recombinant Anhui/1 virus possessing Shanghai/1-NA-294K (c, f). At 2 h after infection, mice were treated with oseltamivir phosphate, zanamivir, laninamivir octanoate, favipiravir, or PBS and distilled water. PBS served as a control for intranasal administration; distilled water served as a control for oral administration. Body weights were monitored daily (a–c). Three mice per group were euthanized at 3 and 6 d.p.i., and the virus titres in lungs were determined by plaque assays in MDCK cells (d–f). Statistically significant differences between virus titres of control mice and those of mice treated with antiviral drugs were determined by using Welch’s t-test or Student’s t-test on the result of the F-test. The resulting P values were corrected by using Holm’s method (*P < 0.05).**Virus was not recovered from all three animals infected with CA04 virus (for the 104 p.f.u. infection groups, the virus titres for the individual animals were 102.0 p.f.u. ml−1 at 3 d.p.i. and 102.2 and 103.1 p.f.u. ml−1 at 6 d.p.i. For the 103 p.f.u. infection groups, the virus titres for the individual animals were 102.2 and 101.8 p.f.u. ml−1 at 3 d.p.i.). Error bars denote standard deviations.
In summary, on the basis of their sequences and phylogenetic relationships, Anhui/1 and Shanghai/1 originated from an avian host but possess several characteristic features of human influenza viruses, such as efficient binding to human-type receptors, efficient replication in mammalian cells (probably conferred by PB2-627K) and respiratory droplet transmission in ferrets (Anhui/1). These properties, together with the low efficacy of NA inhibitors and the lack of human immunity (we tested 500 human sera collected from various age groups in Japan and found no antibodies that recognized Anhui/1), make A(H7N9) viruses a formidable threat to public health.
Methods Summary
Viruses
A(H7N9) viruses were propagated in embryonated chicken eggs to produce viral stocks. Control viruses were propagated as described in Methods. All experiments with A(H7N9) viruses were carried out in approved biosafety level (BSL3) containment laboratories.
Animals
All animals were used according to approved protocols for their care and use. Detailed procedures are provided in Methods.
Antiviral sensitivity of viruses in mice
Six-week-old female BALB/c mice (Japan SLC; groups of six) were intranasally inoculated with 103 or 104 p.f.u. of Anhui/1, CA04 or a recombinant virus possessing the Shanghai/1 NA gene encoding NA-294K and the remaining genes from Anhui/1. At 2 h post-infection, mice were administered antiviral compounds as described in detail in Methods. Three mice per group were euthanized at 3 or 6 d.p.i. and the virus titres in lungs were determined by plaque assays in MDCK cells.
Online Methods
Viruses
Anhui/1 and Shanghai/1, both kindly provided by Y. Shu, and Dk/GM466 were propagated in embryonated chicken eggs. For antigenic characterization by haemagglutination inhibition assays (see below for detailed procedures), Anhui/1 was also propagated in MDCK cells. CA04, Kawasaki/173 and Vietnam/1203 were propagated in MDCK cells. All experiments with H7N9 viruses were performed in enhanced biosafety level 3 (BSL3) containment laboratories at the University of Tokyo and the National Institute of Infectious Diseases, Japan, which are approved for such use by the Ministry of Agriculture, Forestry and Fisheries, Japan; or in enhanced BSL3 containment laboratories at the University of Wisconsin-Madison, which are approved for such use by the Centers for Disease Control and Prevention and by the US Department of Agriculture.
Cells
MDCK cells were maintained in Eagle’s MEM containing 5% newborn calf serum. Human embryonic kidney 293T cells were maintained in DMEM containing 10% FCS. Normal human bronchial epithelial cells (NHBEs) were obtained from Lonza. The monolayers of NHBE cells were cultured and differentiated as previously described29. All cells were incubated at 37 °C with 5% CO2.
Antiviral compounds
Laninamivir and laninamivir octanoate were kindly provided by Daiichi Sankyo Co. Favipiravir was kindly provided by Toyama Chemical Co. and oseltamivir carboxylate was provided by F. Hoffmann-La Roche. Zanamivir was kindly provided by GlaxoSmithKline. Peramivir was kindly provided by Shionogi & Co.
Reverse genetics
Plasmid-based reverse genetics for influenza virus generation was performed as previously described30. In brief, plasmids encoding the complementary DNAs for the eight viral RNA segments under the control of the human RNA polymerase I promoter and the mouse RNA polymerase I terminator (referred to as PolI plasmids), and plasmids for the expression of the viral PB2, PB1, PA and nucleoprotein proteins derived from a laboratory-adapted influenza A virus strain A/WSN/33 (H1N1), under the control of the chicken β-actin promoter31, were transfected into 293T cells with the help of a transfection reagent, Trans-IT 293 (Mirus). At 48 h post-transfection, culture supernatants were collected and inoculated to embryonated chicken eggs for virus propagation.
Growth kinetics of virus in cell culture
MDCK cells were infected in triplicate in 12-well plates with Anhui/1, Dk/GM466 or CA04 at a multiplicity of infection (m.o.i.) of 0.01. After incubation at 37 °C for 1 h, the viral inoculum was replaced with MEM containing 0.3% bovine serum albumin (with 0.75 μg ml−1 trypsin treated with l-1-tosylamide-2- phenylethyl chloromethyl ketone), followed by further incubation at 37 °C. Culture supernatants collected at the indicated time points were subjected to virus titration by using plaque assays in MDCK cells.
Cultures of differentiated NHBE cells grown on semipermeable membrane supports were washed extensively with DMEM to remove accumulated mucus and infected in triplicate with virus at a m.o.i. of 0.001 from the apical surface. The inoculum was removed after 30 min of incubation at 33 °C or 37 °C, and cells were further incubated at 33 °C or 37 °C. Samples were collected at 6, 12, 24, 48, 72 and 96 h post-infection from the apical surface. Apical collection was performed by adding 500 μl of medium to the apical surface, followed by incubation for 30 min at 33 °C or 37 °C, and removal of the medium from the apical surface. The titres of viruses released into the cell culture supernatant were determined by plaque assay in MDCK cells.
Animal experiments
The sample sizes (n = 3) for the mouse, ferret, quail and chicken studies were chosen because they have previously been shown to be sufficient to evaluate a significant difference among groups10,14,32,33,34. For the nonhuman primate and pig experiments, two or three animals per group were used and no statistical analysis was performed. No method of randomization was used to determine how animals were allocated to the experimental groups and processed in this study. The investigator was not blinded to the group allocation during the experiments or when assessing the outcome.
Experimental infection of mice
Six-week-old female BALB/c mice (Japan SLC) were used in this study. Baseline body weights were measured before infection. Under anaesthesia, four mice per group were intranasally inoculated with 101, 102, 103, 104, 105 or 106 p.f.u. (50 μl) of Anhui/1, Shanghai/1, Dk/GM466 or CA04. Body weight and survival were monitored daily for 14 days. For virological and pathological examinations, six mice per group were intranasally infected with 104 or 106 p.f.u. (50 μl) of the viruses and three mice per group were euthanized at 3 and 6 d.p.i. The virus titres in various organs were determined by plaque assays in MDCK cells. All experiments with mice were performed in accordance with the University of Tokyo’s Regulations for Animal Care and Use and approved by the Animal Experiment Committee of the Institute of Medical Science, the University of Tokyo.
Experimental infection of ferrets
Five- to eight-month-old female ferrets (Triple F Farms), which were serologically negative by haemagglutination inhibition assay for currently circulating human influenza viruses, were used in this study. Under anaesthesia, six ferrets per group were intranasally inoculated with 106 p.f.u. (0.5 ml) of Anhui/1, Shanghai/1, Dk/GM466 or CA04. Three ferrets per group were euthanized at 3 and 6 d.p.i. for virological and pathological examinations. The virus titres in various organs were determined by plaque assays in MDCK cells. All experiments with ferrets were performed in accordance with the University of Tokyo’s Regulations for Animal Care and Use and approved by the Animal Experiment Committee of the Institute of Medical Science, the University of Tokyo.
Ferret transmission study
Pairs of ferrets were individually housed in adjacent wireframe cages that prevented direct and indirect contact between animals but allowed spread of influenza virus by respiratory droplets. Under anaesthesia, three ferrets per group were intranasally inoculated with 0.5 ml of 106 p.f.u. ml−1 of Anhui/1, Dk/GM466 or CA04 (inoculated ferrets). One day after infection, three naive ferrets (contact ferrets) were each placed in a cage adjacent to an infected ferret (in these cages, infected and contact ferrets are separated by ∼5 cm). Body weight and temperature were monitored every other day. Nasal washes were collected from infected ferrets on day 1 after inoculation and from contact ferrets on day 1 after co-housing, and then every other day (for up to 9 days) for virological examinations. The virus titres in nasal washes were determined by plaque assays in MDCK cells.
Experimental infection of cynomolgus macaques
Approximately 2-year-old male cynomolgus macaques (Macaca fascicularis) from Cambodia (obtained from Japan Laboratory Animals), weighing 2.2–2.8 kg and serologically negative by AniGen AIV antibody ELISA, which detects infection of all influenza A virus subtypes (Animal Genetics) and neutralization against A/Osaka/1365/2009 (H1N1pdm09), A/Kawasaki/UTK-4/2009 (seasonal H1N1), A/Kawasaki/UTK-20/2008 (H3N2), B/Tokyo/UT-E2/2008 (type B) and A/duck/Hong Kong/301/78 (H7N2) viruses, were used in this study. Under anaesthesia, six and four macaques were inoculated with Anhui/1 or Dk/GM466 (107 p.f.u. ml−1 each), respectively, through a combination of intratracheal (4.5 ml), intranasal (0.5 ml per nostril), ocular (0.1 ml per eye) and oral (1 ml) routes (resulting in a total infectious dose of 6.7 × 107 p.f.u.). Body temperature was monitored at 0, 1, 3, 5 and 6 d.p.i. by rectal thermometer. Nasal and tracheal swabs were collected at 1, 3, 5 and 6 d.p.i. for virological examinations. Three Anhui/1- and two Dk/GM466-infected macaques per group were euthanized at 3 and 6 d.p.i. for virological and pathological examinations. Virus titres were determined by plaque assays in MDCK cells. All experiments with macaques were performed in accordance with the Regulation on Animal Experimentation Guidelines at Kyoto University (5 February, 2007) and were approved by the Committee for Experimental Use of Nonhuman Primates in the Institute for Virus Research, Kyoto University.
Experimental infection of miniature pigs
Two- to three-month-old female specific-pathogen-free miniature pigs (NIBS line; Nippon Institute for Biological Science), which were serologically negative by neutralization assay for currently circulating human and swine influenza viruses, were used in this study. Baseline body temperatures were measured before infection. Four and two pigs were intranasally inoculated with 107 p.f.u. (1 ml) of Anhui/1 or Dk/GM466, respectively. Body temperature was monitored daily. Nasal swabs were collected every day for virological examinations. Two pigs per group were euthanized at 3 d.p.i. for virological and pathological examinations; the remaining two Anhui/1-inoculated pigs were euthanized at 6 d.p.i. Virus titres were determined by plaque assays in MDCK cells. All experiments with miniature pigs were performed in accordance with guidelines established by the Animal Experiment Committee of the Graduate School of Veterinary Medicine, Hokkaido University, and were approved by the Institutional Animal Care and Use Committee of Hokkaido University.
Experimental infection of chickens
Four-week-old female specific-pathogen-free chickens (Nisseiken Co.) were used in this study. Six chickens per group were intranasally inoculated with 2 × 106 p.f.u. (0.2 ml) of Anhui/1 or Dk/GM466. Tracheal and cloacal swabs were collected every day for virological examinations. Three chickens per group were euthanized at 3 and 6 d.p.i. for virological examinations. The virus titres in various organs and swabs were determined by plaque assays in MDCK cells. All experiments with chickens were performed in accordance with the Animal Experimentation Guidelines of the National Institute of Infectious Disease (NIID) and were approved by the Animal Care and Use Committee of the NIID.
Experimental infection of quails
Four-month-old female quails (Yamanaka Koucho En) were used in this study. Six quails per group were intranasally inoculated with 2 × 106 p.f.u. (0.2 ml) of Anhui/1 or Dk/GM466. Tracheal and cloacal swabs were collected every day for virological examinations. Three quails per group were euthanized at 3 and 6 d.p.i. for virological examinations. The virus titres in various organs and swabs were determined by plaque assays in MDCK cells. All experiments with quails were performed in accordance with the University of Tokyo's Regulations for Animal Care and Use and were approved by the Animal Experiment Committee of the Institute of Medical Science, the University of Tokyo.
Pathological examination
Excised tissues of animal organs preserved in 10% phosphate-buffered formalin were processed for paraffin embedding and cut into 3-μm-thick sections. One section from each tissue sample was stained using a standard haematoxylin and eosin procedure, whereas another one was processed for immunohistological staining with a rabbit polyclonal antibody for type A influenza nucleoprotein antigen (prepared in our laboratory) that reacts comparably with all of the viruses tested in this study. Specific antigen–antibody reactions were visualized with 3,3′-diaminobenzidine tetrahydrochloride staining by using the DAKO LSAB2 system (DAKO Cytomation).
Cytokine and chemokine measurement
Macaque lung homogenates and serum samples were processed with the MILLIPLEX MAP Non-human Primate Cytokine/Chemokine Panel–Premixed 23-Plex (Merck Millipore). Array analysis was performed by using the Bio-Plex Protein Array system (Bio-Rad Laboratories).
NA inhibition assay
In vitro NA activity of viruses was determined by using the commercially available NA-Fluor Influenza Neuraminidase Assay Kit (Applied Biosystems). In brief, diluted viruses were mixed with the indicated amounts of oseltamivir carboxylate, zanamivir, laninamivir or peramivir and incubated at 37 °C for 30 min. Methylumbelliferyl-N-acetylneuraminic acid (MUNANA) was then added as a fluorescent substrate, and the mixture was incubated at 37 °C for 1 h. The reaction was stopped by adding 0.12 M Na2CO3 in 40% ethanol. The fluorescence of the solution was measured at an excitation wavelength of 355 nm and an emission wavelength of 460 nm, and the IC50 values were calculated.
Polymerase inhibitor sensitivity assay
8 × 105 MDCK cells were infected with approximately 50 p.f.u. of viruses. After incubation at 37 °C for 1 h, the viral inoculum was replaced with agarose medium containing various concentrations of favipiravir. After the cells were incubated at 37 °C for 2 days, plaques were visualized by crystal violet staining and counted.
Antiviral sensitivity of viruses in mice
Under anaesthesia, six mice per group were intranasally inoculated with 103 or 104 p.f.u. (50 μl) of Anhui/1, CA04 or a recombinant virus possessing the Shanghai/1 NA gene encoding NA-294K and the remaining genes from Anhui/1. At 2 h after inoculation, mice were treated with the following antiviral compounds: (1) oseltamivir phosphate: 4 or 40 mg per kg per 200 μl, administered orally twice a day for 5 days; (2) zanamivir: 0.8 or 8 mg per kg per 50 μl, administered intranasally once daily for 5 days; (3) laninamivir: 0.75 mg per kg per 50 μl, administered intranasally once during the entire experimental course; (4) favipiravir: 30 or 150 mg per kg per 200 μl, administered orally twice a day for 5 days; (5) or PBS intranasally (50 μl) and distilled water orally administered. For virological examinations, three mice per group were euthanized at 3 and 6 d.p.i. The virus titres in lungs were determined by plaque assays in MDCK cells.
Antigenicity characterization by HI assays
Anti-H7 HA monoclonal antibodies 46/6, 46/2 and 55/3 against A/seal/Massachusetts/1/80 (H7N7) virus were kindly provided by R. G. Webster. The goat polyclonal antibody NR-9226 (raised against A/Netherlands/219/2003 (H7N7)) was obtained from BEI Resources. The remaining antibodies, that is, mouse monoclonal antibodies B1275m and B1275m (raised against A/Netherlands/219/2003 (H7N7), MyBioSource), 127-10023 (raised against A/FPV/Rostock/34 (H7N1), RayBiotech), 10H9, 9A9 and 1H11 (raised against A/FPV/Rostock/34 (H7N1), HyTest), and rabbit polyclonal antibody MBS432028 (raised against A/chicken/MD/MINHMA/2004 (H7N2), MyBioSource) were commercially available. Anhui/1 propagated in embryonated chicken eggs or in MDCK cells was used in this study. Antibodies were serially diluted twofold with PBS in 96-well U-bottom microtitre plates and mixed with the amount of virus equivalent to eight haemagglutination units, followed by incubation at room temperature (25 °C) for 30 min. After adding 50 μl of 0.5% turkey red blood cells, the mixtures were gently mixed and incubated at room temperature for a further 45 min. haemagglutination inhibition titres are expressed as the inverse of the highest antibody dilution that inhibited haemagglutination (Supplementary Table 18). These data were used to select antibodies for glycan arrays.
Serology with human sera
Human sera, collected in Japan in November 2012 from 200 donors ranging in age from 20 to 63 years, were treated with receptor-destroying enzyme (Denka Seiken Co). Twofold serial dilutions of the treated sera were mixed with 100 p.f.u. of Anhui/1 and incubated at 37 °C for 1 h. MDCK cells were inoculated with the virus-serum mixtures and cultured for 3 days. The neutralizing activity of the sera was determined based on the cytopathic effects in inoculated cells. All experiments with human sera were approved by the Research Ethics Review Committee of the Institute of Medical Science, the University of Tokyo (approval number: 21-38-1117).
A total of 300 serum samples were also obtained from the serum bank of the National Institute of Infectious Diseases in Japan. Samples were collected from different regions of Japan during 2010–2011. Subjects were divided into 10 age groups, 30 samples per group, and analysed for antibodies against Anhui/1 by use of the haemagglutination inhibition assay with 0.5% turkey red blood cells.
Glycan arrays
Glycan array analysis was performed on a glass slide microarray containing 6 replicates of 57 diverse sialic acid-containing glycans including terminal sequences and intact N-linked and O-linked glycans found on mammalian and avian glycoproteins and glycolipids35. β-propiolactone-inactivated viruses containing A(H7N9) virus HA and NA genes in the background of A/Puerto Rico/8/34 (H1N1) virus were applied to the array at dilutions of 128–512 haemagglutination units ml−1. After incubation at room temperature for 1 h, slides were washed and overlaid with a 1:200 dilution of rabbit or goat anti-H7 antibody for 1 h (selected on the basis of antibody characterization with several H7 viruses; see Supplementary Table 18), and finally with anti-rabbit IgG Alexa Fluor 488 or anti-goat IgG Alexa Fluor 647 (Invitrogen) at 10 μg ml−1. Slides were then washed and scanned on a ProScanArray Express HT (PerkinElmer) confocal slide scanner to detect bound virus. A complete list of glycans present on the array is provided in Supplementary Materials (Supplementary Table 9).
Electron microscopy
Anhui/1 was inoculated into 10-day-old embryonated chicken eggs and the allantoic membranes were collected 24 h after inoculation. They were then processed for ultrathin section electron microscopy and scanning electron microscopy as described previously10,36.
Statistical analysis
All statistical analyses were performed using JMP Pro 9.0.2 (SAS Institute). Statistically significant differences between the virus titres of Dk/GM466-infected mice and those of other mice were determined by using Welch’s t-test with Bonferroni’s correction. Comparisons of virus titres in antiviral sensitivity assays in mice were also done using Welch’s t-test or Student’s t-test on the result of the F-test. The resulting P values were corrected by using the Holm’s method.
Biosafety and biosecurity
All recombinant DNA protocols were approved by the University of Wisconsin-Madison’s Institutional Biosafety Committee after risk assessments were conducted by the Office of Biological Safety, and by the University of Tokyo’s Subcommittee on Living Modified Organisms, and, when required, by the competent minister of Japan. In addition, the University of Wisconsin-Madison Biosecurity Task Force regularly reviews the research program and ongoing activities of the laboratory. The task force has a diverse skill set and provides support in the areas of biosafety, facilities, compliance, security and health. Members of the Biosecurity Task Force are in frequent contact with the principal investigator and laboratory personnel to provide oversight and assure biosecurity. All experiments with H7N9 viruses were performed in enhanced BSL3 containment laboratories. Ferret transmission studies were conducted by two scientists with DVM and/or PhD degrees that each had more than 5 years of experience working with highly pathogenic influenza viruses and performing animal studies with such viruses. Our staff who work with ferrets, nonhuman primates, pigs, chickens and quails wear disposable overalls and powered air-purifying respirators that filter the air. Biosecurity monitoring of the facilities is ongoing. All personnel complete rigorous biosafety, BSL3 and Select Agent (for the US laboratory) training before participating in BSL3-level experiments. The principal investigator participates in training sessions and emphasizes compliance to maintain safe operations and a responsible research environment. The laboratory occupational health plans are in compliance with policies at the respective institutions (University of Wisconsin-Madison and the University of Tokyo Occupational Health Programs).
References
World Health Organization China–WHO Joint Mission on Human Infection with Avian Influenza A(H7N9) Virus. 18–24 April 2013, Mission Report. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/ChinaH7N9JointMissionReport2013.pdf (2013)
Gao, R. et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 368, 1888–1897 (2013)
World Health Organization Number of confirmed human cases of avian influenza A(H7N9) reported to WHO. Report 8 – data in WHO/HQ as of 30 May 2013, 15:45 GMT+1. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/08_ReportWebH7N9Number.pdf (2013)
Kageyama, T. et al. Genetic analysis of novel avian A(H7N9) influenza viruses isolated from patients in China, February to April 2013. Euro Surveill. 18, 20453 (2013)
Liu, Q. et al. Genomic signature and protein sequence analysis of a novel influenza A (H7N9) virus that causes an outbreak in humans in China. Microbes Infect. (2013)
Rogers, G. N. et al. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature 304, 76–78 (1983)
Hatta, M., Gao, P., Halfmann, P. & Kawaoka, Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293, 1840–1842 (2001)
Subbarao, E. K., Kawaoka, Y. & Murphy, B. R. Rescue of an influenza A virus wild-type PB2 gene and a mutant derivative bearing a site-specific temperature-sensitive and attenuating mutation. J. Virol. 67, 7223–7228 (1993)
Li, Z. et al. Molecular basis of replication of duck H5N1 influenza viruses in a mammalian mouse model. J. Virol. 79, 12058–12064 (2005)
Itoh, Y. et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460, 1021–1025 (2009)
van den Brand, J. M. et al. Severity of pneumonia due to new H1N1 influenza virus in ferrets is intermediate between that due to seasonal H1N1 virus and highly pathogenic avian influenza H5N1 virus. J. Infect. Dis. 201, 993–999 (2010)
Min, J. Y. et al. Classical swine H1N1 influenza viruses confer cross protection from swine-origin 2009 pandemic H1N1 influenza virus infection in mice and ferrets. Virology 408, 128–133 (2010)
Maines, T. R. et al. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 325, 484–487 (2009)
Munster, V. J. et al. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science 325, 481–483 (2009)
Imai, M. et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486, 420–428 (2012)
Jackson, S. et al. Reassortment between avian H5N1 and human H3N2 influenza viruses in ferrets: a public health risk assessment. J. Virol. 83, 8131–8140 (2009)
Maines, T. R. et al. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc. Natl Acad. Sci. USA 103, 12121–12126 (2006)
Herfst, S. et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336, 1534–1541 (2012)
Yamada, S. et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 444, 378–382 (2006)
Chutinimitkul, S. et al. In vitro assessment of attachment pattern and replication efficiency of H5N1 influenza A viruses with altered receptor specificity. J. Virol. 84, 6825–6833 (2010)
Srinivasan, K., Raman, R., Jayaraman, A., Viswanathan, K. & Sasisekharan, R. Quantitative characterization of glycan-receptor binding of H9N2 influenza A virus hemagglutinin. PLoS ONE 8, e59550 (2013)
Chandrasekaran, A. et al. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nature Biotechnol. 26, 107–113 (2008)
Chen, L. M. et al. In vitro evolution of H5N1 avian influenza virus toward human-type receptor specificity. Virology 422, 103–113 (2012)
Belser, J. A. et al. Contemporary North American influenza H7 viruses possess human receptor specificity: implications for virus transmissibility. Proc. Natl Acad. Sci. USA 105, 7558–7563 (2008)
McKimm-Breschkin, J. L. et al. Mutations in a conserved residue in the influenza virus neuraminidase active site decreases sensitivity to Neu5Ac2en-derived inhibitors. J. Virol. 72, 2456–2462 (1998)
Centers for Disease Control & Prevention Emergence of avian influenza A(H7N9) virus causing severe human illness - China, February–April 2013. MMWR Morb. Mortal. Wkly Rep. 62, 366–371 (2013)
Wetherall, N. T. et al. Evaluation of neuraminidase enzyme assays using different substrates to measure susceptibility of influenza virus clinical isolates to neuraminidase inhibitors: report of the neuraminidase inhibitor susceptibility network. J. Clin. Microbiol. 41, 742–750 (2003)
Liu, X. et al. Poor responses to oseltamivir treatment in a patient with influenza A (H7N9) virus infection. Emerg. Microbes & Infections 2, e27 (2013)
Jakiela, B., Brockman-Schneider, R., Amineva, S., Lee, W. M. & Gern, J. E. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am. J. Respir. Cell Mol. Biol. 38, 517–523 (2008)
Neumann, G. et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl Acad. Sci. USA 96, 9345–9350 (1999)
Niwa, H., Yamamura, K. & Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 (1991)
Makarova, N. V., Ozaki, H., Kida, H., Webster, R. G. & Perez, D. R. Replication and transmission of influenza viruses in Japanese quail. Virology 310, 8–15 (2003)
Liu, J. et al. Highly pathogenic H5N1 influenza virus infection in migratory birds. Science 309, 1206 (2005)
Zhu, H. et al. Infectivity, transmission, and pathology of human H7N9 influenza in ferrets and pigs. Science http://dx.doi.org/10.1126/science.1239844 (2013)
Xu, R. et al. A recurring motif for antibody recognition of the receptor-binding site of influenza hemagglutinin. Nature Struct. Mol. Biol. 20, 363–370 (2013)
Noda, T. et al. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature 439, 490–492 (2006)
Acknowledgements
We thank Y. Shu for A/Anhui/1/2013 (H7N9) and A/Shanghai/1/2013 (H7N9) viruses. We thank the IMSUT serum bank for providing human sera. We thank R. Webster for providing monoclonal antibody to A/seal/Massachusetts/1/80 (H7N7). Polyclonal anti-influenza virus H7 HA, A/Netherlands/219/2003 (H7N7) (anti-serum, goat) NR-9226, was obtained through the National Institutes of Health (NIH) Biodefense and Emerging Infections Research Resources Repository, National Institute of Allergy and Infectious Diseases (NIAID), NIH. We thank S. Watson for editing the manuscript, T. Suzuki, K. Takahashi, S. Fujisaki and H. Xu for discussions, and Y. Sato, H. Sugawara, A. Sato, M. Ejima and T. Miura for technical assistance. We thank Toyama Chemical Co. for providing favipiravir, Daiichi Sankyo Co. for providing laninamivir, F. Hoffmann-La Roche for providing oseltamivir carboxylate, GlaxoSmithKline for providing zanamivir and Shionogi & Co. for providing peramivir. This work was supported by the Japan Initiative for Global Research Network on Infectious Diseases from the Ministry of Education, Culture, Sports, Science and Technology, Japan; by grants-in-aid from the Ministry of Health, Labour and Welfare, Japan; by ERATO (Japan Science and Technology Agency); by NIAID Public Health Service research grants AI099274 and AI058113 to J.C.P., and by an NIAID-funded Center for Research on Influenza Pathogenesis (CRIP, HHSN266200700010C) to Y.K.
Author information
Authors and Affiliations
Contributions
T.W., M.K., S. Fukuyama, M. Imai, S. Yamada, S.M., S. Yamayoshi, K.I.-H., Y. Sakoda, E.T., M.H., S.W., E.A.M., G.N., H.K., T.O., J.C.P., M.T. and Y.K. designed the study; T.W., M.K., S. Fukuyama, N.N., M. Imai, S. Yamada, S.M., S. Yamayoshi, K.I.-H., Y. Sakoda, E.T., R.M., T.N., M.H., H.I., D.Z., N.K., M.S., R.P.d.V., S.S., M. Okamatsu., T.T., Y.T., N.F., K.G., H.K., I.I., M. Ito, Y.S.-T., Y. Sugita, R.U., R.Y., A.J.E., G.Z., S. Fang, J.P., A.H., Y.U., T.S. and H.H. performed the experiments; T.W., M.K., S. Fang, N.N., M. Imai, S. Yamayoshi, S.M., S. Yamada, K.I.-H., Y. Sakoda, E.T., R.M., T.N., M.H., H.I., D.Z., R.P.V., S.S., T.T., Y.T., H.K., E.K. and H. H. analysed the data; T.W., S. Fukuyama, N.N., E.T., R.M., M.H., R.P.d.V., M. Ozawa, G.N., T.O., J.C.P., H.H., M.T. and Y.K. wrote the manuscript; Y.K. oversaw the project. T.W., M.K., S. Fukuyama, N.N. and M. Imai contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
Y.K. has received speaker’s honoraria from Chugai Pharmaceuticals, Novartis, Daiichi-Sankyo Pharmaceutical, Toyama Chemical, Wyeth, GlaxoSmithKline and Astellas; grant support from Chugai Pharmaceuticals, Daiichi Sankyo Pharmaceutical, Toyama Chemical and Otsuka Pharmaceutical Co.; is a consultant for Crucell; and is a founder of FluGen. G.N. is a founder of FluGen.
Supplementary information
Supplementary Information
This file contains Supplementary Results, Supplementary Figures S1-S11, Supplementary Tables S1-S18 and a Supplementary Reference. (PDF 7391 kb)
Rights and permissions
About this article
Cite this article
Watanabe, T., Kiso, M., Fukuyama, S. et al. Characterization of H7N9 influenza A viruses isolated from humans. Nature 501, 551–555 (2013). https://doi.org/10.1038/nature12392
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12392
This article is cited by
-
Attenuation of A(H7N9) influenza virus infection in mice exposed to cigarette smoke
npj Viruses (2024)
-
Pathogen change of avian influenza virus in the live poultry market before and after vaccination of poultry in southern China
Virology Journal (2021)
-
Avian influenza A (H7N9) virus: from low pathogenic to highly pathogenic
Frontiers of Medicine (2021)
-
Transmission of SARS-CoV-2 virus and ambient temperature: a critical review
Environmental Science and Pollution Research (2021)
-
Pulmonary endothelium-derived PD-L1 induced by the H9N2 avian influenza virus inhibits the immune response of T cells
Virology Journal (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.