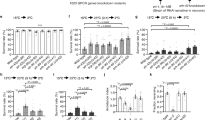
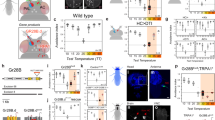
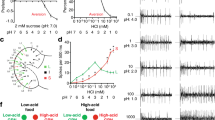
Abstract
Behavioural responses to temperature are critical for survival, and animals from insects to humans show strong preferences for specific temperatures1,2. Preferred temperature selection promotes avoidance of adverse thermal environments in the short term and maintenance of optimal body temperatures over the long term1,2, but its molecular and cellular basis is largely unknown. Recent studies have generated conflicting views of thermal preference in Drosophila, attributing importance to either internal3 or peripheral4 warmth sensors. Here we reconcile these views by showing that thermal preference is not a singular response, but involves multiple systems relevant in different contexts. We found previously that the transient receptor potential channel TRPA1 acts internally to control the slowly developing preference response of flies exposed to a shallow thermal gradient3. We now find that the rapid response of flies exposed to a steep warmth gradient does not require TRPA1; rather, the gustatory receptor GR28B(D) drives this behaviour through peripheral thermosensors. Gustatory receptors are a large gene family, widely studied in insect gustation and olfaction, and are implicated in host-seeking by insect disease vectors5,6,7, but have not previously been implicated in thermosensation. At the molecular level, GR28B(D) misexpression confers thermosensitivity upon diverse cell types, suggesting that it is a warmth sensor. These data reveal a new type of thermosensory molecule and uncover a functional distinction between peripheral and internal warmth sensors in this tiny ectotherm reminiscent of thermoregulatory systems in larger, endothermic animals2. The use of multiple, distinct molecules to respond to a given temperature, as observed here, may facilitate independent tuning of an animal’s distinct thermosensory responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
19 August 2013
The sentence starting: ‘To quantify the thermosensitivity...’ was corrected in the HTML.
References
Garrity, P. A., Goodman, M. B., Samuel, A. D. & Sengupta, P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 24, 2365–2382 (2010)
Flouris, A. D. Functional architecture of behavioural thermoregulation. Eur. J. Appl. Physiol. 111, 1–8 (2011)
Hamada, F. N. et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220 (2008)
Gallio, M., Ofstad, T. A., Macpherson, L. J., Wang, J. W. & Zuker, C. S. The coding of temperature in the Drosophila brain. Cell 144, 614–624 (2011)
Thorne, N., Chromey, C., Bray, S. & Amrein, H. Taste perception and coding in Drosophila. Curr. Biol. 14, 1065–1079 (2004)
Vosshall, L. B. & Stocker, R. F. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 30, 505–533 (2007)
Silbering, A. F. & Benton, R. Ionotropic and metabotropic mechanisms in chemoreception: ‘chance or design’? EMBO Rep. 11, 173–179 (2010)
Viswanath, V. et al. Opposite thermosensor in fruitfly and mouse. Nature 423, 822–823 (2003)
Foelix, R. F., Stocker, R. F. & Steinbrecht, R. A. Fine structure of a sensory organ in the arista of Drosophila melanogaster and some other dipterans. Cell Tissue Res. 258, 277–287 (1989)
Thorne, N. & Amrein, H. Atypical expression of Drosophila gustatory receptor genes in sensory and central neurons. J. Comp. Neurol. 506, 548–568 (2008)
Robertson, H. M., Warr, C. G. & Carlson, J. R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 100 (suppl. 2). 14537–14542 (2003)
Sayeed, O. & Benzer, S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc. Natl Acad. Sci. USA 93, 6079–6084 (1996)
Sweeney, S. T., Broadie, K., Keane, J., Niemann, H. & O’Kane, C. J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14, 341–351 (1995)
Zars, T. Two thermosensors in Drosophila have different behavioral functions. J. Comp. Physiol. A 187, 235–242 (2001)
Kim, S. H. et al. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl Acad. Sci. USA 107, 8440–8445 (2010)
Kang, K. et al. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature 481, 76–80 (2012)
Pulver, S. R., Pashkovski, S. L., Hornstein, N. J., Garrity, P. A. & Griffith, L. C. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J. Neurophysiol. 101, 3075–3088 (2009)
Vyklický, L. et al. Temperature coefficient of membrane currents induced by noxious heat in sensory neurones in the rat. J. Physiol. (Lond.) 517, 181–192 (1999)
Bernstein, J. G., Garrity, P. A. & Boyden, E. S. Optogenetics and thermogenetics: technologies for controlling the activity of targeted cells within intact neural circuits. Curr. Opin. Neurobiol. 22, 61–71 (2012)
Gingl, E., Hinterwirth, A. & Tichy, H. Sensory representation of temperature in mosquito warm and cold cells. J. Neurophysiol. 94, 176–185 (2005)
Sato, K., Tanaka, K. & Touhara, K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc. Natl Acad. Sci. USA 108, 11680–11685 (2011)
Liu, J. et al. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nature Neurosci. 13, 715–722 (2010)
Xiang, Y. et al. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468, 921–926 (2010)
Wang, G. et al. Anopheles gambiae TRPA1 is a heat-activated channel expressed in thermosensitive sensilla of female antennae. Eur. J. Neurosci. 30, 967–974 (2009)
Cho, H. et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nature Neurosci. 15, 1015–1021 (2012)
Xiao, B., Coste, B., Mathur, J. & Patapoutian, A. Temperature-dependent STIM1 activation induces Ca2+ influx and modulates gene expression. Nature Chem. Biol. 7, 351–358 (2011)
Kang, K. et al. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 464, 597–600 (2010)
Suchard, M. A. & Redelings, B. D. BAli-Phy: simultaneous Bayesian inference of alignment and phylogeny. Bioinformatics 22, 2047–2048 (2006)
Zhong, L. et al. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP channel. Cell Rep. 1, 43–55 (2012)
Redelings, B. D. & Suchard, M. A. Incorporating indel information into phylogeny estimation for rapidly emerging pathogens. BMC Evol. Biol. 7, 40 (2007)
Le, S. Q. & Gascuel, O. An improved general amino acid replacement matrix. Mol. Biol. Evol. 25, 1307–1320 (2008)
Acknowledgements
We thank the Garrity laboratory, C. B. Chien, L. Huang, R. Huey, E. Marder and G. Turrigiano for comments, H. Amrein, Y. Jan, C. Montell, C. Zuker and Bloomington Stock Center for strains, N. Donelson, M. Klein, A. Samuel, T. Lauer, P. Taneja, S. Nelson, Y. Yu, H. Bell, P. Sengupta, F. Baier and L. Vosshall for assistance. Supported by grants from National Institute of Mental Health (NIMH) (EUREKA R01 MH094721), National Institute of Neurological Disorders and Stroke (NINDS) (PO1 NS044232) and National Science Foundation (IOS-1025307) to P.A.G., National Institute of General Medical Sciences (NIGMS) (R01 GM054408) and NIMH (R01 MH067284) to L.C.G., NIGMS (R01 GM094468) to D.L.T. and NINDS National Research Service Award to V.C.P.
Author information
Authors and Affiliations
Contributions
L.N., P.B., A.M.L., L.C.G. and P.A.G. designed experiments. L.N. performed molecular genetics, behaviour and chemosensor electrophysiology. P.B. performed neuromuscular electrophysiology. E.C.C., A.M.L. and J.O.F. performed behaviour electrophysiology. V.C.P. performed chemosensor electrophysiology. D.L.T. and P.A.G. performed bioinformatics. L.N., P.B., D.L.T., L.C.G. and P.A.G. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-9. (PDF 10684 kb)
Warmth-triggered incapacitation of Actin>Gr28b(D) flies
Flies that express Gr28b(D) broadly (Actin-Gal4;UAS-Gr28b(D)) were temporarily incapacitated by warming, while control flies (containing only the Actin-Gal4 source or the UAS-Gr28b(D) transgene) were not. All flies were heated by submerging their vials in a 37˚C water bath for 180 seconds. Some frames were deleted as indicated. The video is accelerated 8-fold. (MOV 6085 kb)
Rights and permissions
About this article
Cite this article
Ni, L., Bronk, P., Chang, E. et al. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature 500, 580–584 (2013). https://doi.org/10.1038/nature12390
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12390
This article is cited by
-
Genetic atlas of hygro-and thermosensory cells in the vinegar fly Drosophila melanogaster
Scientific Reports (2023)
-
Pheromone sensing in Drosophila requires support cell-expressed Osiris 8
BMC Biology (2022)
-
Comparative analysis of temperature preference behavior and effects of temperature on daily behavior in 11 Drosophila species
Scientific Reports (2022)
-
Robustness and plasticity in Drosophila heat avoidance
Nature Communications (2021)
-
Thermoresponsive motor behavior is mediated by ring neuron circuits in the central complex of Drosophila
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.