Abstract
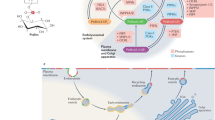
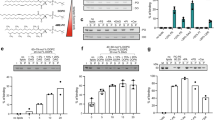
Phosphorylated sphingolipids ceramide-1-phosphate (C1P) and sphingosine-1-phosphate (S1P) have emerged as key regulators of cell growth, survival, migration and inflammation1,2,3,4,5. C1P produced by ceramide kinase is an activator of group IVA cytosolic phospholipase A2α (cPLA2α), the rate-limiting releaser of arachidonic acid used for pro-inflammatory eicosanoid production3,6,7,8,9, which contributes to disease pathogenesis in asthma or airway hyper-responsiveness, cancer, atherosclerosis and thrombosis. To modulate eicosanoid action and avoid the damaging effects of chronic inflammation, cells require efficient targeting, trafficking and presentation of C1P to specific cellular sites. Vesicular trafficking is likely10 but non-vesicular mechanisms for C1P sensing, transfer and presentation remain unexplored11,12. Moreover, the molecular basis for selective recognition and binding among signalling lipids with phosphate headgroups, namely C1P, phosphatidic acid or their lyso-derivatives, remains unclear. Here, a ubiquitously expressed lipid transfer protein, human GLTPD1, named here CPTP, is shown to specifically transfer C1P between membranes. Crystal structures establish C1P binding through a novel surface-localized, phosphate headgroup recognition centre connected to an interior hydrophobic pocket that adaptively expands to ensheath differing-length lipid chains using a cleft-like gating mechanism. The two-layer, α-helically-dominated ‘sandwich’ topology identifies CPTP as the prototype for a new glycolipid transfer protein fold13 subfamily. CPTP resides in the cell cytosol but associates with the trans-Golgi network, nucleus and plasma membrane. RNA interference-induced CPTP depletion elevates C1P steady-state levels and alters Golgi cisternae stack morphology. The resulting C1P decrease in plasma membranes and increase in the Golgi complex stimulates cPLA2α release of arachidonic acid, triggering pro-inflammatory eicosanoid generation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Accessions
Protein Data Bank
Data deposits
Atomic coordinates and structure factors for human CPTP crystal complexes with various lipids andmouse apo-CPTP have been deposited in the Protein Data Bank. Accession codes are: 2:0-C1P–CPTP (4K80), 8:0-C1P–CPTP (4KF6), 12:0-C1P–CPTP (4K85), 16:0-C1P–CPTP (4K84), 18:1-C1P–CPTP (4K8N), di12:0-PA–CPTP (4KBS) and mouse apo-CPTP (4KBR).
References
Hannun, Y. A. & Obeid, L. M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature Rev. Mol. Cell Biol. 9, 139–150 (2008)
Chalfant, C. E. & Spiegel, S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J. Cell Sci. 118, 4605–4612 (2005)
Lamour, N. F. & Chalfant, C. E. Ceramide kinase and the ceramide-1-phosphate/cPLA2α interaction as a therapeutic target. Curr. Drug Targets 9, 674–682 (2008)
Gangoiti, P. et al. Control of metabolism and signaling of simple bioactive sphingolipids: Implications in disease. Prog. Lipid Res. 49, 316–334 (2010)
Maceyka, M., Harikumar, K. B., Milstien, S. & Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 22, 50–60 (2012)
Wymann, M. P. & Schneiter, R. Lipid signalling in disease. Nature Rev. Mol. Cell Biol. 9, 162–176 (2008)
Leslie, C. C., Gangelhoff, T. A. & Gelb, M. H. Localization and function of cytosolic phospholipase A2α at the Golgi. Biochimie 92, 620–626 (2010)
Bechler, M. E., de Figueiredo, P. & Brown, W. J. A. PLA1–2 punch regulates the Golgi complex. Trends Cell Biol. 22, 116–124 (2012)
Harizi, H., Corcuff, J. B. & Gualde, N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol. Med. 14, 461–469 (2008)
Boath, A. et al. Regulation and traffic of ceramide 1-phosphate produced by ceramide kinase: comparative analysis to glucosylceramide and sphingomyelin. J. Biol. Chem. 283, 8517–8526 (2008)
Lev, S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nature Rev. Mol. Cell Biol. 11, 739–750 (2010)
Prinz, W. A. Lipid trafficking sans vesicles: where, why, how? Cell 143, 870–874 (2010)
Malinina, L., Malakhova, M. L., Teplov, A., Brown, R. E. & Patel, D. J. Structural basis for glycosphingolipid transfer specificity. Nature 430, 1048–1053 (2004)
Malakhova, M. L. et al. Point mutational analysis of the liganding site in human glycolipid transfer protein. Functionality of the complex. J. Biol. Chem. 280, 26312–26320 (2005)
Hirsch, A. K., Fischer, F. R. & Diederich, F. Phosphate recognition in structural biology. Angew. Chem. Int. Ed. Engl. 46, 338–352 (2007)
Bourquin, F., Riezman, H., Capitani, G. & Grutter, M. G. Structure and function of sphingosine-1-phosphate lyase, a key enzyme of sphingolipid metabolism. Structure 18, 1054–1065 (2010)
Berna, A. et al. For whom the bell tolls? DING proteins in health and disease. Cell. Mol Life Sci. 66, 2205–2218 (2009)
Furuhashi, M. & Hotamisligil, G. S. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nature Rev. Drug Discov. 7, 489–503 (2008)
Roderick, S. L. et al. Structure of human phosphatidylcholine transfer protein in complex with its ligand. Nature Struct. Biol. 9, 507–511 (2002)
Hanada, K. et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature 426, 803–809 (2003)
Kudo, N. et al. Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc. Natl Acad. Sci. USA 105, 488–493 (2008)
Zou, X. et al. Human glycolipid transfer protein (GLTP) genes: organization, transcriptional status and evolution. BMC Genomics 9, 72 (2008)
Orengo, C. A. & Thornton, J. M. Protein families and their evolution - a structural perspective. Annu. Rev. Biochem. 74, 867–900 (2005)
Galperin, M. Y. & Koonin, E. V. Divergence and convergence in enzyme evolution. J. Biol. Chem. 287, 21–28 (2012)
Liberles, D. A. et al. The interface of protein structure, protein biophysics, and molecular evolution. Protein Sci. 21, 769–785 (2012)
Lamour, N. F. et al. Ceramide 1-phosphate is required for the translocation of group IVA cytosolic phospholipase A2 and prostaglandin synthesis. J. Biol. Chem. 284, 26897–26907 (2009)
Lamour, N. F. et al. Ceramide kinase uses ceramide provided by ceramide transport protein: localization to organelles of eicosanoid synthesis. J. Lipid Res. 48, 1293–1304 (2007)
Bornancin, F. Ceramide kinase: the first decade. Cell. Signal. 23, 999–1008 (2011)
Durcan, T. M. et al. Tektin 2 is required for central spindle microtubule organization and the completion of cytokinesis. J. Cell Biol. 181, 595–603 (2008)
Samygina, V. R. et al. Enhanced selectivity for sulfatide by engineered human glycolipid transfer protein. Structure 19, 1644–1654 (2011)
Manders, E. M. M., Stap, J., Brakenhoff, G. J., van Driel, R. & Aten, J. A. Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J. Cell Sci. 103, 857–862 (1992)
Hornick, J. E. et al. Amphiastral mitotic spindle assembly in vertebrate cells lacking centrosomes. Curr. Biol. 21, 598–605 (2011)
Wijesinghe, D. S. et al. Use of high performance liquid chromatography-electrospray ionization-tandem mass spectrometry for the analysis of ceramide-1-phosphate levels. J. Lipid Res. 51, 641–651 (2010)
Blaho, V. A., Buczynski, M. W., Brown, C. R. & Dennis, E. A. Lipidomic analysis of dynamic eicosanoid responses during the induction and resolution of Lyme arthritis. J. Biol. Chem. 284, 21599–21612 (2009)
Acknowledgements
This research was supported by NIH/NCI CA121493 (D.J.P. & R.E.B.), NIH/NIGMS GM45928 (R.E.B.), NIH/NIGMS GM072754 (E.H.H.), NIH/CA154314 (C.E.C.), VA Merit Award (C.E.C.), VA Research Career Scientist Award (C.E.C.), VA Career Devel. Award (D.S.W.), NRS-T32/NIGMS 008695 (D.S.W.), Spanish Ministerio de Ciencia e Innovacion BFU2010-17711 (L.M.), Russian Foundation for Basic Research #12-04-00168 (J.G.M.), Hormel Foundation. (R.E.B.), Abby Rockefeller Mauze Trust (D.J.P.) and Maloris Foundation (D.J.P.). We thank H. Pike for expressing and purifying protein used for transfer activity analyses, K. Karanjeet for preparing cells for confocal and epifluorescence microscopy, and the staff of X-29 beamline at the National Synchrotron Light Source and ID-24-C/E beamlines at the Advanced Photon Source for help.
Author information
Authors and Affiliations
Contributions
D.K.S. carried out all structural analyses and provided evidence for C1P binding by CPTP, generated all CPTP point mutants and wrote text. R.K.K did transfer analyses of wild-type CPTP and CPTP point mutants and wrote text. D.S.W. conducted siRNA CPTP knockdown, rescue, and all lipid analyses and wrote text. Xi.Zo. cloned wild-type CPTP and did PCR analyses of CPTP transcript distribution in human tissues. Xi.Zh. did CPTP transfer rate analyses. S.K.M. prepared CPTP RNAi constructs for microscopy and CPTP overexpression constructs for lipidomics analyses. J.G.M. synthesized all fluorescent lipids. L.M. contributed to structural data interpretation. E.H.H. did fluorescence microscopy of CPTP localization in fixed and living cells and finalized the write-up. C.E.C. directed siRNA knockdown, rescue and related lipidomics analyses and finalized the write-up. D.J.P. directed CPTP structural analyses and finalized the write-up. R.E.B. directed functional analyses after the initial CPTP discovery in his laboratory, finalized the write-up and coordinated and integrated all section write-ups.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Results and Discussion, Supplementary references, Supplementary Tables 1-5 and Supplementary Figures 1-14. (PDF 2736 kb)
Rights and permissions
About this article
Cite this article
Simanshu, D., Kamlekar, R., Wijesinghe, D. et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature 500, 463–467 (2013). https://doi.org/10.1038/nature12332
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12332
This article is cited by
-
Bioactive lipid screening during respiratory tract infections with bacterial and viral pathogens in mice
Metabolomics (2022)
-
Crosstalks between inflammasome and autophagy in cancer
Journal of Hematology & Oncology (2020)
-
Placental Production of Eicosanoids and Sphingolipids in Women Who Developed Preeclampsia on Low-Dose Aspirin
Reproductive Sciences (2020)
-
The Cross-Talk Between Sphingolipids and Insulin-Like Growth Factor Signaling: Significance for Aging and Neurodegeneration
Molecular Neurobiology (2019)
-
The vaginal microbiome and preterm birth
Nature Medicine (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.