Abstract
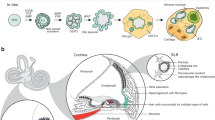
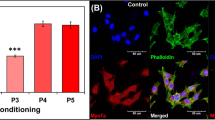
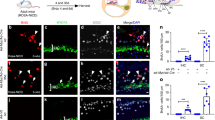
The inner ear contains sensory epithelia that detect head movements, gravity and sound. It is unclear how to develop these sensory epithelia from pluripotent stem cells, a process that will be critical for modelling inner ear disorders or developing cell-based therapies for profound hearing loss and balance disorders1,2. So far, attempts to derive inner ear mechanosensitive hair cells and sensory neurons have resulted in inefficient or incomplete phenotypic conversion of stem cells into inner-ear-like cells3,4,5,6,7. A key insight lacking from these previous studies is the importance of the non-neural and preplacodal ectoderm, two critical precursors during inner ear development8,9,10,11. Here we report the stepwise differentiation of inner ear sensory epithelia from mouse embryonic stem cells (ESCs) in three-dimensional culture12,13. We show that by recapitulating in vivo development with precise temporal control of signalling pathways, ESC aggregates transform sequentially into non-neural, preplacodal and otic-placode-like epithelia. Notably, in a self-organized process that mimics normal development, vesicles containing prosensory cells emerge from the presumptive otic placodes and give rise to hair cells bearing stereocilia bundles and a kinocilium. Moreover, these stem-cell-derived hair cells exhibit functional properties of native mechanosensitive hair cells and form specialized synapses with sensory neurons that have also arisen from ESCs in the culture. Finally, we demonstrate how these vesicles are structurally and biochemically comparable to developing vestibular end organs. Our data thus establish a new in vitro model of inner ear differentiation that can be used to gain deeper insight into inner ear development and disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bermingham-McDonogh, O. & Reh, T. A. Regulated reprogramming in the regeneration of sensory receptor cells. Neuron 71, 389–405 (2011)
Brigande, J. V. & Heller, S. Quo vadis, hair cell regeneration? Nature Neurosci. 12, 679–685 (2009)
Ouji, Y., Ishizaka, S., Nakamura-Uchiyama, F. & Yoshikawa, M. In vitro differentiation of mouse embryonic stem cells into inner ear hair cell-like cells using stromal cell conditioned medium. Cell Death Dis. 3, e314 (2012)
Oshima, K. et al. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell 141, 704–716 (2010)
Kondo, T. et al. Tlx3 exerts context-dependent transcriptional regulation and promotes neuronal differentiation from embryonic stem cells. Proc. Natl Acad. Sci. USA 105, 5780–5785 (2008)
Reyes, J. H. et al. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J. Neurosci. 28, 12622–12631 (2008)
Chen, W. et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature 490, 278–282 (2012)
Schlosser, G. Induction and specification of cranial placodes. Dev. Biol. 294, 303–351 (2006)
Pieper, M., Ahrens, K., Rink, E., Peter, A. & Schlosser, G. Differential distribution of competence for panplacodal and neural crest induction to non-neural and neural ectoderm. Development 139, 1175–1187 (2012)
Groves, A. K. & Fekete, D. M. Shaping sound in space: the regulation of inner ear patterning. Development 139, 245–257 (2012)
Grocott, T., Tambalo, M. & Streit, A. The peripheral sensory nervous system in the vertebrate head: a gene regulatory perspective. Dev. Biol. 370, 3–23 (2012)
Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011)
Suga, H. et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature 480, 57–62 (2011)
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008)
Nakano, T. et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785 (2012)
Kamiya, D. et al. Intrinsic transition of embryonic stem-cell differentiation into neural progenitors. Nature 470, 503–509 (2011)
Wilson, P. A. & Hemmati-Brivanlou, A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature 376, 331–333 (1995)
Bernardo, A. S. et al. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell 9, 144–155 (2011)
Chambers, S. M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nature Biotechnol. 27, 275–280 (2009)
Kwon, H.-J., Bhat, N., Sweet, E. M., Cornell, R. A. & Riley, B. B. Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet. 6, e1001133 (2010)
Kwon, H.-J. & Riley, B. B. Mesendodermal signals required for otic induction: Bmp-antagonists cooperate with Fgf and can facilitate formation of ectopic otic tissue. Dev. Dyn. 238, 1582–1594 (2009)
Ladher, R. K., O’Neill, P. & Begbie, J. From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development 137, 1777–1785 (2010)
Laine, H., Sulg, M., Kirjavainen, A. & Pirvola, U. Cell cycle regulation in the inner ear sensory epithelia: role of cyclin D1 and cyclin-dependent kinase inhibitors. Dev. Biol. 337, 134–146 (2010)
Li, A., Xue, J. & Peterson, E. H. Architecture of the mouse utricle: macular organization and hair bundle heights. J. Neurophysiol. 99, 718–733 (2008)
Meyers, J. R. et al. Lighting up the senses: FM1–43 loading of sensory cells through nonselective ion channels. J. Neurosci. 23, 4054–4065 (2003)
Géléoc, G. S. G., Risner, J. R. & Holt, J. R. Developmental acquisition of voltage-dependent conductances and sensory signaling in hair cells of the embryonic mouse inner ear. J. Neurosci. 24, 11148–11159 (2004)
Oesterle, E. C., Campbell, S., Taylor, R. R., Forge, A. & Hume, C. R. Sox2 and Jagged1 expression in normal and drug-damaged adult mouse inner ear. J. Assoc. Res. Otolaryngol. 9, 65–89 (2008)
Warchol, M. E. & Richardson, G. P. Expression of the Pax2 transcription factor is associated with vestibular phenotype in the avian inner ear. Dev. Neurobiol. 69, 191–202 (2009)
Desai, S. S., Zeh, C. & Lysakowski, A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J. Neurophysiol. 93, 251–266 (2005)
Lysakowski, A. et al. Molecular microdomains in a sensory terminal, the vestibular calyx ending. J. Neurosci. 31, 10101–10114 (2011)
Ying, Q.-L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008)
Eiraku, M. & Sasai, Y. Mouse embryonic stem cell culture for generation of three-dimensional retinal and cortical tissues. Nature Protocols 7, 69–79 (2012)
Koehler, K. R. et al. Extended passaging increases the efficiency of neural differentiation from induced pluripotent stem cells. BMC Neurosci. 12, 82 (2011)
Hama, H. et al. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nature Neurosci. 14, 1481–1488 (2011)
Jegalian, B. G. & De Robertis, E. M. Homeotic transformations in the mouse induced by overexpression of a human Hox3. 3 transgene. Cell 71, 901–910 (1992)
Coate, T. M. et al. Otic mesenchyme cells regulate spiral ganglion axon fasciculation through a Pou3f4/EphA4 signaling pathway. Neuron 73, 49–63 (2012)
Gale, J. E., Marcotti, W., Kennedy, H. J., Kros, C. J. & Richardson, G. P. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J. Neurosci. 21, 7013–7025 (2001)
Hu, Z. & Corwin, J. T. Inner ear hair cells produced in vitro by a mesenchymal-to-epithelial transition. Proc. Natl Acad. Sci. 104, 16675–16680 (2007)
Acknowledgements
The authors would like to thank G. Oxford for contributing unpublished data and discussion; E. Beans, G. Kamocka, K. Dunn and C. Miller for technical assistance; P. Dolle, R. Romand, J. Williams, J. Meyer, X. Zhang and T. Cummins for comments and discussion; E. Tobin, R. Meadows, J. Hamilton, S. Majumdar and G. Wagner for editorial assistance. This work was supported by National Institutes of Health (NIH) grants RC1DC010706, R21DC012617 and R01GM086544. K.R.K. was supported by a Paul and Carole Stark Neurosciences Fellowship and an Indiana Clinical and Translational Science Institute Predoctoral Fellowship (NIH TL1RR025759). A.I.M. was supported by NIH R01MH52619 (awarded to A. Shekhar).
Author information
Authors and Affiliations
Contributions
K.R.K. conceived and designed the study, performed experiments, analysed data, created the figures and wrote the manuscript. A.M.M. and D.P. performed experiments and analysed data. A.I.M. generated electrophysiological data. E.H. helped to design the study, provided financial support, monitored the experiments and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion, Supplementary Figures 1-16 and additional references. (PDF 20951 kb)
3D reconstruction of a vesicle lined with Myo7a+ Sox2+ hair cells and Sox2+ supporting cells
A series of images and an animated 3D reconstruction of the vesicle seen in Figure 3. (MOV 12719 kb)
Characteristics of sensory epithelia displayed in vesicles containing hair cells
A series of images highlighting (1) the F-actin+ banding pattern on the luminal surface of each hair cell vesicle and (2) the F-actin+ Myo7a+ stereocilia-like protrusions emanating from the apical surface of hair cells into the lumen. Additionally, a 3D reconstruction of the sensory epithelium rotates in order to provide multiple views of the protruding stereocilia at day 20. A second 3D reconstruction of an epithelium at day 24 is stained for F-actin and acetylated-α- Tubulin to visualize more mature stereocilia bundles and kinocilium, respectively. (MOV 28835 kb)
Rights and permissions
About this article
Cite this article
Koehler, K., Mikosz, A., Molosh, A. et al. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 500, 217–221 (2013). https://doi.org/10.1038/nature12298
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12298
This article is cited by
-
Stem Cell-Based Hair Cell Regeneration and Therapy in the Inner Ear
Neuroscience Bulletin (2024)
-
Organoide – der Schlüssel zu neuen Therapien für das Innenohr?
HNO (2023)
-
Extracting multiple surfaces from 3D microscopy images in complex biological tissues with the Zellige software tool
BMC Biology (2022)
-
Retinoic acid and FGF10 promote the differentiation of pluripotent stem cells into salivary gland placodes
Stem Cell Research & Therapy (2022)
-
CHD7 regulates otic lineage specification and hair cell differentiation in human inner ear organoids
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.