Abstract
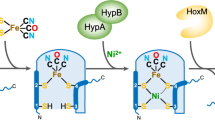
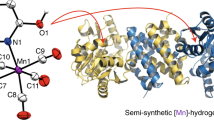
Hydrogenases are the most active molecular catalysts for hydrogen production and uptake1,2, and could therefore facilitate the development of new types of fuel cell3,4,5. In [FeFe]-hydrogenases, catalysis takes place at a unique di-iron centre (the [2Fe] subsite), which contains a bridging dithiolate ligand, three CO ligands and two CN– ligands6,7. Through a complex multienzymatic biosynthetic process, this [2Fe] subsite is first assembled on a maturation enzyme, HydF, and then delivered to the apo-hydrogenase for activation8. Synthetic chemistry has been used to prepare remarkably similar mimics of that subsite1, but it has failed to reproduce the natural enzymatic activities thus far. Here we show that three synthetic mimics (containing different bridging dithiolate ligands) can be loaded onto bacterial Thermotoga maritima HydF and then transferred to apo-HydA1, one of the hydrogenases of Chlamydomonas reinhardtii algae. Full activation of HydA1 was achieved only when using the HydF hybrid protein containing the mimic with an azadithiolate bridge, confirming the presence of this ligand in the active site of native [FeFe]-hydrogenases9,10. This is an example of controlled metalloenzyme activation using the combination of a specific protein scaffold and active-site synthetic analogues. This simple methodology provides both new mechanistic and structural insight into hydrogenase maturation and a unique tool for producing recombinant wild-type and variant [FeFe]-hydrogenases, with no requirement for the complete maturation machinery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tard, C. & Pickett, C. J. Structural and functional analogues of the active sites of the [Fe]-, [NiFe]-, and [FeFe]-hydrogenases. Chem. Rev. 109, 2245–2274 (2009)
Cracknell, J. A., Vincent, K. A. & Armstrong, F. A. Enzymes as working or inspirational electrocatalysts for fuel cells and electrolysis. Chem. Rev. 108, 2439–2461 (2008)
Hambourger, M. et al. [FeFe]-hydrogenase-catalyzed H2 production in a photoelectrochemical biofuel cell. J. Am. Chem. Soc. 130, 2015–2022 (2008)
Krishnan, S. & Armstrong, F. A. Order-of-magnitude enhancement of an enzymatic hydrogen-air fuel cell based on pyrenyl carbon nanostructures. Chem. Sci. 3, 1015–1023 (2012)
Ciaccafava, A. et al. An innovative powerful and mediatorless H2/O2 biofuel cell based on an outstanding bioanode. Electrochem. Commun. 23, 25–28 (2012)
Peters, J. W., Lanzilotta, W. N., Lemon, B. J. & Seefeldt, L. C. X-ray crystal structure of the Fe-only hydrogenase (Cpl) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 282, 1853–1858 (1998)
Nicolet, Y., Piras, C., Legrand, P., Hatchikian, C. E. & Fontecilla-Camps, J. C. Desulfovibrio desulfuricans iron hydrogenase: the structure shows unusual coordination to an active site Fe binuclear center. Structure 7, 13–23 (1999)
Mulder, D. W. et al. Stepwise [FeFe]-hydrogenase H-cluster assembly revealed in the structure of HydAΔEFG. Nature 465, 248–251 (2010)
Nicolet, Y. et al. Crystallographic and FTIR spectroscopic evidence of changes in Fe coordination upon reduction of the active site of the Fe-only hydrogenase from Desulfovibrio desulfuricans. J. Am. Chem. Soc. 123, 1596–1601 (2001)
Silakov, A., Wenk, B., Reijerse, E. & Lubitz, W. 14N HYSCORE investigation of the H-cluster of [FeFe] hydrogenase: evidence for a nitrogen in the dithiol bridge. Phys. Chem. Chem. Phys. 11, 6592–6599 (2009)
Le Cloirec, A. et al. A di-iron dithiolate possessing structural elements of the carbonyl/cyanide sub-site of the H-centre of Fe-only hydrogenase. Chem. Commun. 2285–2286 (1999)
Lyon, E. J., Georgakaki, I. P., Reibenspies, J. H. & Darensbourg, M. Y. Carbon monoxide and cyanide ligands in a classical organometallic complex model for Fe-only hydrogenase. Angew. Chem. Int. Edn Engl. 38, 3178–3180 (1999)
Schmidt, M., Contakes, S. M. & Rauchfuss, T. B. First generation analogues of the binuclear site in the Fe-only hydrogenases: [Fe2(µ-SR)2(CO)4(CN)2 ]2–. J. Am. Chem. Soc. 121, 9736–9737 (1999)
Li, H. X. & Rauchfuss, T. B. Iron carbonyl sulfides, formaldehyde, and amines condense to give the proposed azadithiolate cofactor of the Fe-only hydrogenases. J. Am. Chem. Soc. 124, 726–727 (2002)
Song, L. C., Yang, Z. Y., Bian, H. Z. & Hu, Q. M. Novel single and double diiron oxadithiolates as models for the active site of [Fe]-only hydrogenases. Organometallics 23, 3082–3084 (2004)
Pandey, A. S., Harris, T. V., Giles, L. J., Peters, J. W. & Szilagyi, R. K. Dithiomethylether as a ligand in the hydrogenase H-cluster. J. Am. Chem. Soc. 130, 4533–4540 (2008)
Brazzolotto, X. et al. The [Fe-Fe]-hydrogenase maturation protein HydF from Thermotoga maritima is a GTPase with an iron-sulfur cluster. J. Biol. Chem. 281, 769–774 (2006)
Adamska, A. et al. Identification and characterization of the “super-reduced” state of the H-cluster in [FeFe] hydrogenase: a new building block for the catalytic cycle? Angew. Chem. Int. Ed. 51, 11458–11462 (2012)
Czech, I., Silakov, A., Lubitz, W. & Happe, T. The [FeFe]-hydrogenase maturase HydF from Clostridium acetobutylicum contains a CO and CN– ligated iron cofactor. FEBS Lett. 584, 638–642 (2010)
Geiss, A. & Vahrenkamp, H. M. (µ-CN)Fe(µ-CN)M' chains with phthalocyanine iron centers: preparation, structures, and isomerization. Inorg. Chem. 39, 4029–4036 (2000)
Hubrich, M., Jeschke, G. & Schweiger, A. The generalized hyperfine sublevel coherence transfer experiment in one and two dimensions. J. Chem. Phys. 104, 2172–2184 (1996)
Gambarelli, S., Luttringer, F., Padovani, D., Mulliez, E. & Fontecave, M. Activation of the anaerobic ribonucleotide reductase by S-adenosylmethionine. ChemBioChem 6, 1960–1962 (2005)
Chen, D. W., Walsby, C., Hoffman, B. M. & Frey, P. A. Coordination and mechanism of reversible cleavage of S-adenosylmethionine by the [4Fe-4S] center in lysine 2,3-aminomutase. J. Am. Chem. Soc. 125, 11788–11789 (2003)
Coronado, E. et al. Pressure-tuning of magnetism and linkage isomerism in iron(II) hexacyanochromate. J. Am. Chem. Soc. 127, 4580–4581 (2005)
Shatruk, M. et al. Properties of Prussian blue materials manifested in molecular complexes: observation of cyanide linkage isomerism and spin-crossover behavior in pentanuclear cyanide clusters. J. Am. Chem. Soc. 129, 6104–6116 (2007)
Happe, T. & Naber, J. D. Isolation, characterization and N-terminal amino-acid-sequence of hydrogenase from the green-alga Chlamydomonas reinhardtii. Eur. J. Biochem. 214, 475–481 (1993)
Kamp, C. et al. Isolation and first EPR characterization of the [FeFe]-hydrogenases from green algae. Biochim. Biophys. Acta Bioenerg. 1777, 410–416 (2008)
Sybirna, K. et al. Shewanella oneidensis: a new and efficient system for expression and maturation of heterologous [Fe-Fe] hydrogenase from Chlamydomonas reinhardtii. BMC Biotechnol. 8, 73–81 (2008)
Darensbourg, M. Y., Lyon, E. J., Zhao, X. & Georgakaki, I. P. The organometallic active site of [Fe] hydrogenase: models and entatic states. Proc. Natl Acad. Sci. USA 100, 3683–3688 (2003)
Mertens, R. & Liese, A. Biotechnological applications of hydrogenases. Curr. Opin. Biotechnol. 15, 343–348 (2004)
Fiedler, A. T. & Brunold, T. C. Combined spectroscopic/computational study of binuclear Fe(I)-Fe(I) complexes: implications for the fully-reduced active-site cluster of Fe-only hydrogenases. Inorg. Chem. 44, 1794–1809 (2005)
Kuchenreuther, J. M. et al. High-yield expression of heterologous [FeFe] hydrogenases in Escherichia coli. PLoS ONE 5, e15491 (2010)
Fish, W. W. Rapid colorimetric micromethod for the quantitation of complexed iron in biological samples. Methods Enzymol. 158, 357–364 (1988)
Beinert, H. Semi-micro methods for analysis of labile sulfide and of labile sulfide plus sulfane sulfur in unusually stable iron sulfur proteins. Anal. Biochem. 131, 373–378 (1983)
Hemschemeier, A., Melis, A. & Happe, T. Analytical approaches to photobiological hydrogen production in unicellular green algae. Photosynth. Res. 102, 523–540 (2009)
Stripp, S. T. et al. How oxygen attacks [FeFe] hydrogenases from photosynthetic organisms. Proc. Natl Acad. Sci. USA 106, 17331–17336 (2009)
Acknowledgements
G.B. acknowledges support from the Bengt Lundqvist Minnesfond, FORMAS (contract number 213-2010-563) and the Swedish Royal Academy of Sciences. This work was supported by the French National Research Agency (ANR) through grant 07-BLAN-0298-01 and the Labex programme (ARCANE, 11-LABX-003). V.A. acknowledges support from the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013/ERC Grant Agreement no. 306398). T.H. was supported by the Deutsche Forschungsgemeinschaft (HA 255/2-1), the BMBF (Bio-H2) and the Volkswagen foundation (LigH2t). A.A., E.R. and W.L. thank the Max Planck Society for financial support, and A. Silakov for providing the FTIR processing software.
Author information
Authors and Affiliations
Contributions
G.B., V.A., M.A., W.L., T.H. and M.F. designed the research; G.B. and T.R.S. prepared and characterized synthetic complexes and hybrid species; C.L., J.E. and G.B. contributed to maturation experiments and H2 evolution measurements; A.A. and C.L. performed FTIR measurements; G.B. and S.G. performed EPR measurements; J.-M.M. did DFT calculations; and M.F., G.B, E.R. and V.A. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Tables1-6, Supplementary Figures 1-7 and Supplementary References. (PDF 1128 kb)
Rights and permissions
About this article
Cite this article
Berggren, G., Adamska, A., Lambertz, C. et al. Biomimetic assembly and activation of [FeFe]-hydrogenases. Nature 499, 66–69 (2013). https://doi.org/10.1038/nature12239
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12239
This article is cited by
-
Photosensitizing metal-organic layers for photocatalysis, artificial photosynthesis and fluorescence imaging
Science China Chemistry (2023)
-
A personal account on 25 years of scientific literature on [FeFe]-hydrogenase
JBIC Journal of Biological Inorganic Chemistry (2023)
-
Biomimetic active sites on monolayered metal–organic frameworks for artificial photosynthesis
Nature Catalysis (2022)
-
Azadithiolate-bridged [FeFe]-hydrogenase mimics with bridgehead N-derivation: structural and electrochemical investigations
Transition Metal Chemistry (2022)
-
Stability of the H-cluster under whole-cell conditions—formation of an Htrans-like state and its reactivity towards oxygen
JBIC Journal of Biological Inorganic Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.