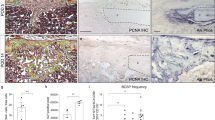
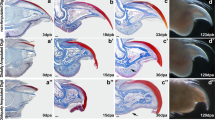
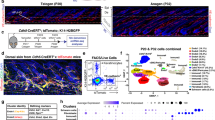
Abstract
The tips of mammalian digits can regenerate after amputation1,2, like those of amphibians. It is unknown why this capacity is limited to the area associated with the nail2,3,4. Here we show that nail stem cells (NSCs) reside in the proximal nail matrix and that the mechanisms governing NSC differentiation are coupled directly with their ability to orchestrate digit regeneration. Early nail progenitors undergo Wnt-dependent differentiation into the nail. After amputation, this Wnt activation is required for nail regeneration and also for attracting nerves that promote mesenchymal blastema growth, leading to the regeneration of the digit. Amputations proximal to the Wnt-active nail progenitors result in failure to regenerate the nail or digit. Nevertheless, β-catenin stabilization in the NSC region induced their regeneration. These results establish a link between NSC differentiation and digit regeneration, and suggest that NSCs may have the potential to contribute to the development of novel treatments for amputees.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Accessions
Gene Expression Omnibus
Data deposits
Expression information has been submitted to the Gene Expression Omnibus database under accession numbers GSE45494, GSM1105640, GSM1105641, GSM1105642 and GSM1105643.
References
Douglas, B. S. Conservative management of guillotine amputation of the finger in children. Aust. Paediatr. J. 8, 86–89 (1972)
Borgens, R. B. Mice regrow the tips of their foretoes. Science 217, 747–750 (1982)
Zhao, W. & Neufeld, D. A. Bone regrowth in young mice stimulated by nail organ. J. Exp. Zool. 271, 155–159 (1995)
Mohammad, K. S., Day, F. A. & Neufeld, D. A. Bone growth is induced by nail transplantation in amputated proximal phalanges. Calcif. Tissue Int. 65, 408–410 (1999)
Rinkevich, Y., Lindau, P., Ueno, H., Longaker, M. T. & Weissman, I. L. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature 476, 409–413 (2011)
Lehoczky, J. A., Robert, B. & Tabin, C. J. Mouse digit tip regeneration is mediated by fate-restricted progenitor cells. Proc. Natl Acad. Sci. USA 108, 20609–20614 (2011)
Neufeld, D. A. Partial blastema formation after amputation in adult mice. J. Exp. Zool. 212, 31–36 (1980)
Han, M., Yang, X., Lee, J., Allan, C. H. & Muneoka, K. Development and regeneration of the neonatal digit tip in mice. Dev. Biol. 315, 125–135 (2008)
Neufeld, D. A. & Zhao, W. Phalangeal regrowth in rodents: postamputational bone regrowth depends upon the level of amputation. Prog. Clin. Biol. Res. 383A, 243–252 (1993)
Norton, L. A. Incorporation of thymidine-methyl-H3 and glycine-2–H3 in the nail matrix and bed of humans. J. Invest. Dermatol. 56, 61–68 (1971)
Fleckman, P., Jaeger, K., Silva, K. A. & Sundberg, J. P. Comparative anatomy of mouse and human nail units. Anat. Rec. (Hoboken) 296, 521–532 (2013)
Al-Qattan, M. M. WNT pathways and upper limb anomalies. J. Hand Surg. Eur. Vol. 36, 9–22 (2011)
Blaydon, D. C. et al. The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nature Genet. 38, 1245–1247 (2006)
Adaimy, L. et al. Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. Am. J. Hum. Genet. 81, 821–828 (2007)
Rabbani, P. et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 145, 941–955 (2011)
Lin, M. H. & Kopan, R. Long-range, nonautonomous effects of activated Notch1 on tissue homeostasis in the nail. Dev. Biol. 263, 343–359 (2003)
Nakamura, M. & Ishikawa, O. The localization of label-retaining cells in mouse nails. J. Invest. Dermatol. 128, 728–730 (2008)
Al Alam, D. et al. Contrasting expression of canonical Wnt signaling reporters TOPGAL, BATGAL and Axin2LacZ during murine lung development and repair. PLoS ONE 6, e23139 (2011)
van de Wetering, M. et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88, 789–799 (1997)
Bänziger, C. et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125, 509–522 (2006)
Zhou, P., Byrne, C., Jacobs, J. & Fuchs, E. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 9, 700–713 (1995)
Lynch, M. H., O'Guin, W. M., Hardy, C., Mak, L. & Sun, T. T. Acidic and basic hair/nail (“hard”) keratins: their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to “soft” keratins. J. Cell Biol. 103, 2593–2606 (1986)
Ducy, P., Zhang, R., Geoffroy, V., Ridall, A. L. & Karsenty, G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747–754 (1997)
Mohammad, K. S. & Neufeld, D. A. Denervation retards but does not prevent toetip regeneration. Wound Repair Regen. 8, 277–281 (2000)
Brockes, J. P. The nerve dependence of amphibian limb regeneration. J. Exp. Biol. 132, 79–91 (1987)
Kantor, D. B. et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 44, 961–975 (2004)
Zhang, Y. et al. Activation of β-catenin signaling programs embryonic epidermis to hair follicle fate. Development 135, 2161–2172 (2008)
Mullen, L. M., Bryant, S. V., Torok, M. A., Blumberg, B. & Gardiner, D. M. Nerve dependency of regeneration: the role of Distal-less and FGF signaling in amphibian limb regeneration. Development 122, 3487–3497 (1996)
Kawakami, Y. et al. Wnt/β-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 20, 3232–3237 (2006)
Yokoyama, H., Ogino, H., Stoick-Cooper, C. L., Grainger, R. M. & Moon, R. T. Wnt/β-catenin signaling has an essential role in the initiation of limb regeneration. Dev. Biol. 306, 170–178 (2007)
Harada, N. et al. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 18, 5931–5942 (1999)
Vasioukhin, V., Degenstein, L., Wise, B. & Fuchs, E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl Acad. Sci. USA 96, 8551–8556 (1999)
Lowry, W. E. et al. Defining the impact of β-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 19, 1596–1611 (2005)
Myung, P. S., Takeo, M., Ito, M. & Atit, R. P. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J. Invest. Dermatol. (2013)
DasGupta, R. & Fuchs, E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557–4568 (1999)
Lustig, B. et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22, 1184–1193 (2002)
Barrandon, Y. & Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl Acad. Sci. USA 84, 2302–2306 (1987)
Yu, L. et al. BMP signalling induces digit regeneration in neonatal mice. Development 137, 551–559 (2010)
Acknowledgements
We thank T. Andl, T. Endo, L. Miller, P. Myung, M. Schober and T. T. Sun for invaluable suggestions and discussion. We thank T. Endo for demonstrating the method of bead implantation. We thank T. T. Sun for the AE13 antibody, K. Muneoka for the Bmp4 plasmid, and A. Mansukhani for 3T3 cells. We thank the Genome Technology Center at NYU (National Institutes of Health (NIH) grant 5P30CA0016087-32 and P30 CA016087-30), and the Center for Functional Genomics at University at Albany for carrying out microarray analyses. We thank F. Liang at the NYU Microscopy Core for transmission electron microscopy (TEM) analysis. We thank the NYU Microscopy Core for the use of a confocal microscope (NCRRS10 RR023704-01A1). M.T. is supported by the NYU Kimmel Stem Cell Center and NYSTEM training grant C026880. M.I. is supported by NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant 1R01AR059768-01A1, the Ellison Medical Foundation and funding from the Department of Dermatology and Cell Biology, and the Helen and Martin Kimmel Center for Stem Cell Biology, at NYU.
Author information
Authors and Affiliations
Contributions
M.T. designed and carried out experiments, interpreted data and wrote the manuscript. W.C.C., P.R. and Q.S. performed experiments and interpreted data. M.M.T. generated β-cateninfl/ex3 mice and interpreted the data. C.L. and W.L. interpreted data. M.I. designed experiments, interpreted data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-18 and Supplementary Table 1. (PDF 5256 kb)
Rights and permissions
About this article
Cite this article
Takeo, M., Chou, W., Sun, Q. et al. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature 499, 228–232 (2013). https://doi.org/10.1038/nature12214
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12214
This article is cited by
-
Deer antler renewal gives insights into mammalian epimorphic regeneration
Cell Regeneration (2023)
-
Current Trends in the Treatment of Traumatic Nail Injuries and the Prospect of Solutions from Regenerative Engineering
Regenerative Engineering and Translational Medicine (2023)
-
Regeneration of the digestive system in the crinoid Lamprometra palmata (Mariametridae, Comatulida)
Cell and Tissue Research (2023)
-
Activation of the Melanocortin-4 receptor signaling by α-MSH stimulates nerve-dependent mouse digit regeneration
Cell Regeneration (2021)
-
Single-cell RNA-seq reveals novel mitochondria-related musculoskeletal cell populations during adult axolotl limb regeneration process
Cell Death & Differentiation (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.