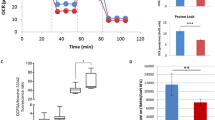
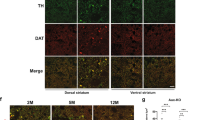
Abstract
Many neurodegenerative disorders, such as Alzheimer’s, Parkinson’s and polyglutamine diseases, share a common pathogenic mechanism: the abnormal accumulation of disease-causing proteins, due to either the mutant protein’s resistance to degradation or overexpression of the wild-type protein. We have developed a strategy to identify therapeutic entry points for such neurodegenerative disorders by screening for genetic networks that influence the levels of disease-driving proteins. We applied this approach, which integrates parallel cell-based and Drosophila genetic screens, to spinocerebellar ataxia type 1 (SCA1), a disease caused by expansion of a polyglutamine tract in ataxin 1 (ATXN1). Our approach revealed that downregulation of several components of the RAS–MAPK–MSK1 pathway decreases ATXN1 levels and suppresses neurodegeneration in Drosophila and mice. Importantly, pharmacological inhibitors of components of this pathway also decrease ATXN1 levels, suggesting that these components represent new therapeutic targets in mitigating SCA1. Collectively, these data reveal new therapeutic entry points for SCA1 and provide a proof-of-principle for tackling other classes of intractable neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Evans, D. A. Estimated prevalence of Alzheimer’s disease in the United States. Milbank Q. 68, 267–289 (1990)
Hindle, J. V. Ageing, neurodegeneration and Parkinson’s disease. Age Ageing 39, 156–161 (2010)
Marsden, C. D. & Parkes, J. D. Success and problems of long-term levodopa therapy in Parkinson’s disease. Lancet 1, 345–349 (1977)
Scarpini, E., Scheltens, P. & Feldman, H. Treatment of Alzheimer’s disease: current status and new perspectives. Lancet Neurol. 2, 539–547 (2003)
Ross, C. A. & Poirier, M. A. Protein aggregation and neurodegenerative disease. Nature Med. 10, S10–S17 (2004)
Taylor, J. P., Hardy, J. & Fischbeck, K. H. Toxic proteins in neurodegenerative disease. Science 296, 1991–1995 (2002)
Haass, C. & Selkoe, D. J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nature Rev. Mol. Cell Biol. 8, 101–112 (2007)
Zoghbi, H. Y. & Orr, H. T. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 23, 217–247 (2000)
Singleton, A. B. et al. α-synuclein locus triplication causes Parkinson’s disease. Science 302, 841 (2003)
Chartier-Harlin, M. C. et al. α-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364, 1167–1169 (2004)
Rovelet-Lecrux, A. et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nature Genet. 38, 24–26 (2006)
Götz, J. & Ittner, L. M. Animal models of Alzheimer’s disease and frontotemporal dementia. Nature Rev. Neurosci. 9, 532–544 (2008)
Williams, A. J. & Paulson, H. L. Polyglutamine neurodegeneration: protein misfolding revisited. Trends Neurosci. 31, 521–528 (2008)
Xia, H. et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nature Med. 10, 816–820 (2004)
Harper, S. Q. et al. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proc. Natl Acad. Sci. USA 102, 5820–5825 (2005)
Yamamoto, A., Lucas, J. J. & Hen, R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell 101, 57–66 (2000)
Zu, T. et al. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J. Neurosci. 24, 8853–8861 (2004)
Kordasiewicz, H. B. et al. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron 74, 1031–1044 (2012)
Zoghbi, H. Y. & Orr, H. T. Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, spinocerebellar ataxia type 1. J. Biol. Chem. 284, 7425–7429 (2009)
Emamian, E. S. et al. Serine 776 of ataxin-1 is critical for polyglutamine-induced disease in SCA1 transgenic mice. Neuron 38, 375–387 (2003)
Noble, M. E., Endicott, J. A. & Johnson, L. N. Protein kinase inhibitors: insights into drug design from structure. Science 303, 1800–1805 (2004)
Fernandez-Funez, P. et al. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 408, 101–106 (2000)
Taniguchi, C. M., Emanuelli, B. & Kahn, C. R. Critial nodes in signalling pathways: insights into insulin action. Nature Rev. Mol. Cell Biol. 7, 85–96 (2006)
Shaharabany, M. et al. Distinct pathways for the involvement of WNK4 in the signaling of hypertonicity and EGF. FEBS J. 275, 1631–1642 (2008)
Teixeira-Castro, A. et al. Neuron-specific proteotoxicity of mutant ataxin-3 in C. elegans: rescue by the DAF-16 and HSF-1 pathways. Hum. Mol. Genet. 20, 2996–3009 (2011)
Cobb, M. H. MAP kinase pathways. Prog. Biophys. Mol. Biol. 71, 479–500 (1999)
Avruch, J. Insulin signal transduction through protein kinase cascades. Mol. Cell. Biochem. 182, 31–48 (1998)
Al-Ramahi, I. et al. dAtaxin-2 mediates expanded Ataxin-1-induced neurodegeneration in a Drosophila model of SCA1. PLoS Genet. 3, e234 (2007)
Jorgensen, N. D. et al. Phosphorylation of ATXN1 at Ser776 in the cerebellum. J. Neurochem. 110, 675–686 (2009)
Roux, P. P. & Blenis, J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68, 320–344 (2004)
Mody, N., Leitch, J., Armstrong, C., Dixon, J. & Cohen, P. Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett. 502, 21–24 (2001)
Lackey, K. et al. The discovery of potent cRaf1 kinase inhibitors. Bioorg. Med. Chem. Lett. 10, 223–226 (2000)
Deak, M., Clifton, A. D., Lucocq, L. M. & Alessi, D. R. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17, 4426–4441 (1998)
Lorenzetti, D. et al. Repeat instability and motor incoordination in mice with a targeted expanded CAG repeat in the Sca1 locus. Hum. Mol. Genet. 9, 779–785 (2000)
Watase, K. et al. A long CAG repeat in the mouse Sca1 locus replicates SCA1 features and reveals the impact of protein solubility on selective neurodegeneration. Neuron 34, 905–919 (2002)
Arthur, J. S. & Cohen, P. MSK1 is required for CREB phosphorylation in response to mitogens in mouse embryonic stem cells. FEBS Lett. 482, 44–48 (2000)
Wiggin, G. R. et al. MSK1 and MSK2 are required for the mitogen- and stress- induced phosphorylation of CREB and ATF1 in fibroblasts. Mol. Cell. Biol. 22, 2871–2881 (2002)
Burright, E. N. et al. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell 82, 937–948 (1995)
Dar, A. C., Das, T. K., Shokat, K. M. & Cagan, R. L. Chemical genetic discovery of targets and anti-targets for cancer polypharmacology. Nature 486, 80–84 (2012)
van Ham, T. J., Breitling, R., Swertz, M. A. & Nollen, E. A. Neurodegenerative diseases: lessons from genome-wide screens in small model organisms. EMBO Mol. Med. 1, 360–370 (2009)
Jeong, H. et al. Acetylation targets mutant huntingtin to autophagosomes for degradation. Cell 137, 60–72 (2009)
Matsumoto, M. et al. Molecular clearance of ataxin-3 is regulated by a mammalian E4. EMBO J. 23, 659–669 (2004)
Cummings, C. J. et al. Mutation of the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine-induced pathology in SCA1 mice. Neuron 24, 879–892 (1999)
Chin, L. & Gray, J. W. Translating insights from the cancer genome into clinical practice. Nature 452, 553–563 (2008)
Falsig, J. & Aguzzi, A. The prion organotypic slice culture assay—POSCA. Nature Protocols 3, 555–562 (2008)
Jafar-Nejad, P., Ward, C. S., Richman, R., Orr, H. T. & Zoghbi, H. Y. Regional rescue of spinocerebellar ataxia type 1 phenotypes by 14–3-3 haploinsufficiency in mice underscores complex pathogenicity in neurodegeneration. Proc. Natl Acad. Sci. USA 108, 2142–2147 (2011)
Bowman, A. B. et al. Duplication of Atxn1l suppresses SCA1 neuropathology by decreasing incorporation of polyglutamine-expanded ataxin-1 into native complexes. Nature Genet. 39, 373–379 (2007)
Lim, J. et al. Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1. Nature 452, 713–718 (2008)
Servadio, A. et al. Expression analysis of the ataxin-1 protein in tissues from normal and spinocerebellar ataxia type 1 individuals. Nature Genet. 10, 94–98 (1995)
Acknowledgements
We thank the members of the Zoghbi, Botas, Orr and Westbrook laboratories for suggestions and discussions, and V. Brandt for editorial input. We also appreciate the help from CCSC and C-BASS cores at The Baylor College of Medicine (BCM) for FACS analysis and the confocal microscopy and mouse behavioural cores of the BCM Intellectual and Developmental Disabilities Research Center (HD024064). Thanks to J. Barrish at Texas Children’s Hospital for help with scanning electron microscopy. This work was supported by a Howard Hughes Medical Institute Collaborative Innovation Awards grant and grant NIH-NS42179 (J.B.) I.A.-R. was supported by an NIH Brain Disorders and Development training grant.
Author information
Authors and Affiliations
Contributions
J.P., I.A.-R., Q.T., N.M., H.T.O., T.F.W., J.B. and H.Y.Z. designed the experiments. J.P., I.A.-R., Q.T., N.M., J.R.D.-G., T.G.-F., H.-C.L., S.L., L.D., H.K., Y.L., P.J.-N., L.S.S., R.R., X.L. and Y.G. performed the research. J.P., I.A.-R., Q.T., N.M., J.R.D.-G., T.G.-F., H.-C.L., S.L., L.D., H.K., Y.L., P.J.-N., C.A.S., J.S.C.A., H.T.O., T.F.W., J.B. and H.Y.Z. analysed and interpreted the data. J.P., I.A.-R., H.T.O., T.F.W., J.B. and H.Y.Z. wrote and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains the N number for Figure 6c in the main paper, Supplementary Figures 1-8, Supplementary Tables 1-4, the genotypes for Drosophila and additional references. (PDF 2136 kb)
Rights and permissions
About this article
Cite this article
Park, J., Al-Ramahi, I., Tan, Q. et al. RAS–MAPK–MSK1 pathway modulates ataxin 1 protein levels and toxicity in SCA1. Nature 498, 325–331 (2013). https://doi.org/10.1038/nature12204
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12204
This article is cited by
-
Intercellular Propagation and Aggregate Seeding of Mutant Ataxin-1
Journal of Molecular Neuroscience (2022)
-
Modifier pathways in polyglutamine (PolyQ) diseases: from genetic screens to drug targets
Cellular and Molecular Life Sciences (2022)
-
DNAzyme Cleavage of CAG Repeat RNA in Polyglutamine Diseases
Neurotherapeutics (2021)
-
Selective neuronal degeneration in MATR3 S85C knock-in mouse model of early-stage ALS
Nature Communications (2020)
-
A kinome-wide RNAi screen identifies ERK2 as a druggable regulator of Shank3 stability
Molecular Psychiatry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.