Abstract
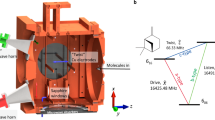
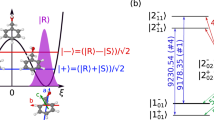
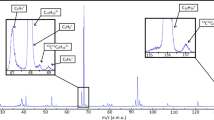
Chirality plays a fundamental part in the activity of biological molecules and broad classes of chemical reactions, but detecting and quantifying it remains challenging1. The spectroscopic methods of choice are usually circular dichroism and vibrational circular dichroism, methods that are forbidden in the electric dipole approximation2. The resultant weak effects produce weak signals, and thus require high sample densities. In contrast, nonlinear techniques probing electric-dipole-allowed effects have been used for sensitive chiral analyses of liquid samples3,4,5,6,7. Here we extend this class of approaches by carrying out nonlinear resonant phase-sensitive microwave spectroscopy of gas phase samples in the presence of an adiabatically switched non-resonant orthogonal electric field; we use this technique to map the enantiomer-dependent sign of an electric dipole Rabi frequency onto the phase of emitted microwave radiation. We outline theoretically how this results in a sensitive and species-selective method for determining the chirality of cold gas-phase molecules, and implement it experimentally to distinguish between the S and R enantiomers of 1,2-propanediol and their racemic mixture. This technique produces a large and definitive signature of chirality, and has the potential to determine the chirality of multiple species in a mixture.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Busch K. W., Busch M. A., eds. Chiral Analysis (Elsevier, 2006)
Fischer, P. & Hache, F. Nonlinear optical spectroscopy of chiral molecules. Chirality 17, 421–437 (2005)
Fischer, P., Wiersma, D. S., Righini, R., Champagne, B. & Buckingham, A. D. Three-wave mixing in chiral liquids. Phys. Rev. Lett. 85, 4253–4256 (2000)
Li, Y. & Bruder, C. Dynamic method to distinguish between left- and right-handed chiral molecules. Phys. Rev. A 77, 015403 (2008)
Rhee, H., Choi, J.-H. & Cho, M. Infrared optical activity: electric field approaches in time domain. Acc. Chem. Res. 43, 1527–1536 (2010)
Hiramatsu, K. et al. Observation of Raman optical activity by heterodyne-detected polarization-resolved coherent anti-Stokes Raman scattering. Phys. Rev. Lett. 109, 083901 (2012)
Hirota, E. Triple resonance for a three-level system of a chiral molecule. Proc. Jpn Acad. B 88, 120–128 (2012)
Townes, C. & Schawlow, A. Microwave Spectroscopy (Dover Publications, 1975)
Darquié, B. et al. Progress toward the first observation of parity violation in chiral molecules by high-resolution laser spectroscopy. Chirality 22, 870–884 (2010)
Quack, M. How important is parity violation for molecular and biomolecular chirality? Angew. Chem. Int. Edn 41, 4618–4630 (2002)
Schnell, M. & Küpper, J. Tailored molecular samples for precision spectroscopy experiments. Faraday Discuss. 150, 33–49 (2011)
Quack, M., Stohner, J. & Willeke, M. High-resolution spectroscopic studies and theory of parity violation in chiral molecules. Annu. Rev. Phys. Chem. 59, 741–769 (2008)
Jacob, A. & Hornberger, K. Effect of molecular rotation on enantioseparation. J. Chem. Phys. 137, 044313 (2012)
Lovas, F. J. et al. Microwave spectrum of 1,2-propanediol. J. Mol. Spectrosc. 257, 82–93 (2009)
Patterson, D. & Doyle, J. M. Cooling molecules in a cell for FTMW spectroscopy. Mol. Phys. 110, 1757–1766 (2012)
Balle, T. J. & Flygare, W. H. Fabry-Perot cavity pulsed Fourier transform microwave spectrometer with a pulsed nozzle particle source. Rev. Sci. Instrum. 52, 33–45 (1981)
Guo, C. et al. Determination of enantiomeric excess in samples of chiral molecules using Fourier transform vibrational circular dichroism spectroscopy: simulation of real-time reaction monitoring. Anal. Chem. 76, 6956–6966 (2004)
Brown, G. G. et al. A broadband Fourier transform microwave spectrometer based on chirped pulse excitation. Rev. Sci. Instrum. 79, 053103 (2008)
Král, P., Thanopulos, I., Shapiro, M. & Cohen, D. Two-step enantio-selective optical switch. Phys. Rev. Lett. 90, 033001 (2003)
Thanopulos, I., Král, P. & Shapiro, M. Theory of a two-step enantiomeric purification of racemic mixtures by optical means: the D2S2 molecule. J. Chem. Phys. 119, 5105–5116 (2003)
Gerbasi, D., Brumer, P., Thanopulos, I., Král, P. & Shapiro, M. Theory of the two step enantiomeric purification of 1,3 dimethylallene. J. Chem. Phys. 120, 11557–11563 (2004)
Pate, B. H. & De Lucia, F. C. (eds) Special issue: Broadband molecular rotational spectroscopy. J. Mol. Spectrosc. 280, 1–2 (2012)
Mata, S., Peña, I., Cabezas, C., López, J. & Alonso, J. A broadband Fourier transform microwave spectrometer with laser ablation source: the rotational spectrum of nicotinic acid. J. Mol. Spectrosc. 280, 91–96 (2012)
Grabow, J.-U. et al. Rapid probe of the nicotine spectra by high-resolution rotational spectroscopy. Phys. Chem. Chem. Phys. 13, 21063–21069 (2011)
Western, C. M. PGOPHER, a Program for Simulating Rotational Structure Version 7.1 (Univ. Bristol, 2010)
Acknowledgements
We thank D. DeMille, A. Vutha and C. Connolly for discussions, and D. DeMille and C. Connolly for contributions to the final manuscript.
Author information
Authors and Affiliations
Contributions
D.P. had the idea for this work, built the apparatus and produced the theoretical simulations. J.M.D. and D.P. developed experimental approaches and designed the apparatus. D.P. and M.S. performed the measurements. All authors wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.P. and J.M.D. declare a financial interest: patents related to this research have been filed by Harvard University. The university’s policy is to share financial rewards from the exploitation of patents with the inventors. M.S. declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Patterson, D., Schnell, M. & Doyle, J. Enantiomer-specific detection of chiral molecules via microwave spectroscopy. Nature 497, 475–477 (2013). https://doi.org/10.1038/nature12150
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12150
This article is cited by
-
Inducing transient enantiomeric excess in a molecular quantum racemic mixture with microwave fields
Nature Communications (2023)
-
Perspective: nanoscale electric sensing and imaging based on quantum sensors
Quantum Frontiers (2023)
-
Effective chirality discrimination via dissipation dynamics
Quantum Information Processing (2023)
-
Circular Dichroism Enhancement Induced by Strong Coupling and Weak Coupling in Metal Disk-Chiral TDBCs-Metal Disk Structure
Plasmonics (2023)
-
Assignment-free chirality detection in unknown samples via microwave three-wave mixing
Communications Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.