Abstract
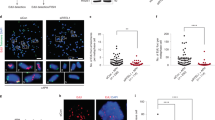
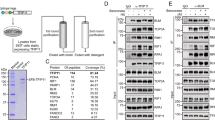
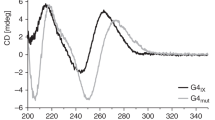
The Saccharomyces cerevisiae Pif1 helicase is the prototypical member of the Pif1 DNA helicase family, which is conserved from bacteria to humans. Here we show that exceptionally potent G-quadruplex unwinding is conserved among Pif1 helicases. Moreover, Pif1 helicases from organisms separated by more than 3 billion years of evolution suppressed DNA damage at G-quadruplex motifs in yeast. The G-quadruplex-induced damage generated in the absence of Pif1 helicases led to new genetic and epigenetic changes. Furthermore, when expressed in yeast, human PIF1 suppressed both G-quadruplex-associated DNA damage and telomere lengthening.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bochman, M. L., Paeschke, K. & Zakian, V. A. DNA secondary structures: stability and function of G-quadruplex structures. Nature Rev. Genet. 13, 770–780 (2012)
Boulé, J.-B., Vega, L. & Zakian, V. The Yeast Pif1p helicase removes telomerase from DNA. Nature 438, 57–61 (2005)
Schulz, V. P. & Zakian, V. A. The Saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell 76, 145–155 (1994)
Zhou, J.-Q., Monson, E. M., Teng, S.-C., Schulz, V. P. & Zakian, V. A. The Pif1p helicase, a catalytic inhibitor of telomerase lengthening of yeast telomeres. Science 289, 771–774 (2000)
Myung, K., Chen, C. & Kolodner, R. D. Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature 411, 1073–1076 (2001)
Paeschke, K., Capra, J. A. & Zakian, V. A. DNA replication through G–quadruplex motifs Is promoted by the Saccharomyces cerevisiae Pif1 DNA helicase. Cell 145, 678–691 (2011)
Lopes, J. et al. G–quadruplex-induced instability during leading-strand replication. EMBO J. 30, 4033–4046 (2011)
Ivessa, A. S., Zhou, J.-Q. & Zakian, V. A. The Saccharomyces Pif1p DNA helicase and the highly related Rrm3p have opposite effects on replication fork progression in ribosomal DNA. Cell 100, 479–489 (2000)
Ivessa, A. S. et al. The Saccharomyces cerevisiae helicase Rrm3p facilitates replication past nonhistone protein-DNA complexes. Mol. Cell 12, 1525–1536 (2003)
Fachinetti, D. et al. Replication termination at eukaryotic chromosomes is mediated by Top2 and occurs at genomic loci containing pausing elements. Mol. Cell 39, 595–605 (2010)
Bochman, M. L., Sabouri, N. & Zakian, V. A. Unwinding the functions of the Pif1 family helicases. DNA Repair (Amst.) 9, 237–249 (2010)
Chisholm, K. M. et al. A genomewide screen for suppressors of Alu-mediated rearrangements reveals a role for PIF1. PLoS ONE 7, e30748 (2012)
Lahaye, A., Leterme, S. & Foury, F. PIF1 DNA helicase from Saccharomyces cerevisiae. Biochemical characterization of the enzyme. J. Biol. Chem. 268, 26155–26161 (1993)
Boulé, J. B. & Zakian, V. A. The yeast Pif1p DNA helicase preferentially unwinds RNA DNA substrates. Nucleic Acids Res. 35, 5809–5818 (2007)
Bochman, M. L., Judge, C. P. & Zakian, V. A. The Pif1 family in prokaryotes: what are our helicases doing in your bacteria? Mol. Biol. Cell 22, 1955–1959 (2011)
Cejka, P. & Kowalczykowski, S. C. The full-length Saccharomyces cerevisiae Sgs1 protein is a vigorous DNA helicase that preferentially unwinds holliday junctions. J. Biol. Chem. 285, 8290–8301 (2010)
Mohaghegh, P., Karow, J. K., Brosh Jr, R. M., Bohr Jr, V. A. & Hickson, I. D. The Bloom’s and Werne’s syndrome proteins are DNA structure-specific helicases. Nucleic Acids Res. 29, 2843–2849 (2001)
Schmidt, K. H., Pennaneach, V., Putnam, C. D. & Kolodner, R. D. Analysis of gross-chromosomal rearrangements in Saccharomyces cerevisiae. Methods Enzymol. 409, 462–476 (2006)
Chen, C. & Kolodner, R. D. Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nature Genet. 23, 81–85 (1999)
Azvolinsky, A., Giresi, P., Lieb, J. & Zakian, V. Highly transcribed RNA polymerase II genes are impediments to replication fork progression in Saccharomyces cerevisiae. Mol. Cell 34, 722–734 (2009)
Smith, S. et al. Mutator genes for suppression of gross chromosomal rearrangements identified by a genome-wide screening in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 101, 9039–9044 (2004)
Gottschling, D. E., Aparicio, O. M., Billington, B. L. & Zakian, V. A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63, 751–762 (1990)
Baur, J. A., Zou, Y., Shay, J. W. & Wright, W. E. Telomere position effect in human cells. Science 292, 2075–2077 (2001)
Mondoux, M. & Zakian, V. in Telomeres 2nd edn (eds de Lange, T., Lundblad, V. & Blackburn, E. H. ) 261–316 (CSHL, 2005)
Piazza, A. et al. Stimulation of gross chromosomal rearrangements by the human CEB1 and CEB25 minisatellites in Saccharomyces cerevisiae depends on G-quadruplexes or Cdc13. PLoS Genet. 8, e1003033 (2012)
Aparicio, O. M., Billington, B. L. & Gottschling, D. E. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell 66, 1279–1287 (1991)
Sarkies, P., Reams, C., Simpson, L. J. & Sale, J. E. Epigenetic instability due to defective replication of structured DNA. Mol. Cell 40, 703–713 (2010)
Ivessa, A. S., Zhou, J.-Q., Schulz, V. P., Monson, E. M. & Zakian, V. A. Saccharomyces Rrm3p, a 5′ to 3′ DNA helicase that promotes replication fork progression through telomeric and sub-telomeric DNA. Genes Dev. 16, 1383–1396 (2002)
Pinter, S. F., Aubert, S. D. & Zakian, V. A. The Schizosaccharomyces pombe Pfh1p DNA helicase is essential for the maintenance of nuclear and mitochondrial DNA. Mol. Cell. Biol. 28, 6594–6608 (2008)
Sikorski, R. S. & Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27 (1989)
Harmon, F. G. & Kowalczykowski, S. C. RecQ helicase, in concert with RecA and SSB proteins, initiates and disrupts DNA recombination. Genes Dev. 12, 1134–1144 (1998)
Capra, J. A., Paeschke, K., Singh, M. & Zakian, V. A. G-quadruplex DNA sequences are evolutionarily conserved and associated with distinct genomic features in Saccharomyces cerevisiae. PLOS Comput. Biol. 6, e1000861 (2010)
Bachrati, C. Z. & Hickson, I. D. Analysis of the DNA unwinding activity of RecQ family helicases. Methods Enzymol. 409, 86–100 (2006)
Wong, I. & Lohman, T. M. A double-filter method for nitrocellulose-filter binding: application to protein-nucleic acid interactions. Proc. Natl Acad. Sci. USA 90, 5428–5432 (1993)
Brosh, R. M., Jr, Opresko, P. L. & Bohr, V. A. Enzymatic mechanism of the WRN helicase/nuclease. Methods Enzymol. 409, 52–85 (2006)
Putnam, C. D. & Kolodner, R. D. Determination of gross chromosomal rearrangement rates. Cold Spring Harbor Protoc. 2010, pdb.prot5492 (2010)
Hall, B. M., Ma, C. X., Liang, P. & Singh, K. K. Fluctuation analysis CalculatOR: a web tool for the determination of mutation rate using Luria–Delbruck fluctuation analysis. Bioinformatics 25, 1564–1565 (2009)
Heid, C. A., Stevens, J., Livak, K. J. & Williams, P. M. Real time quantitative PCR. Genome Res. 6, 986–994 (1996)
Azvolinsky, A., Dunaway, S., Torres, J., Bessler, J. & Zakian, V. A. The S. cerevisiae Rrm3p DNA helicase moves with the replication fork and affects replication of all yeast chromosomes. Genes Dev. 20, 3104–3116 (2006)
Gueldener, U., Heinisch, J., Koehler, G. J., Voss, D. & Hegemann, J. H. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res. 30, e23 (2002)
Longtine, M. S. et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 (1998)
Studier, F. W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 41, 207–234 (2005)
Kushnirov, V. V. Rapid and reliable protein extraction from yeast. Yeast 16, 857–860 (2000)
Runge, K. W. & Zakian, V. A. Introduction of extra telomeric DNA sequences into Saccharomyces cerevisiae results in telomere elongation. Mol. Cell. Biol. 9, 1488–1497 (1989)
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001)
Acknowledgements
We thank J. B. Boule for early work on S. cerevisiae Pif1 biochemistry, P. Opresko for the gift of purified human WRN, E. Allen-Vercoe, K. Bidle, C. Parker, R. Johnson, H. L. Ayala-del-Rio and E. Sockett for materials and for cloning non-yeast Pif1 helicases, and M. Platts for multiplex PCR and Southern analysis methods to characterize GCR clones. We acknowledge financial support from the National Institutes of Health (V.A.Z., S.C.K.), National Science Foundation (K.L.F.), DFG and NJCCR (K.P.) and American Cancer Society (M.L.B.).
Author information
Authors and Affiliations
Contributions
K.P. and M.L.B. purified Pif1 helicases and performed biochemical and GCR experiments; P.D.G. and M.L.B. did the silencing experiments; P.C. purified Sgs1; S.C.K. aided in the analysis and interpretation of the biochemistry and provided purified E. coli RecQ; K.L.F. aided in the analysis and interpretation of GCR events; K.P., M.L.B. and V.A.Z. designed the study, analysed data and wrote the manuscript. K.P. and M.L.B. contributed equally. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text, Supplementary Figures 1-7, Supplementary Tables 1-8 and Supplementary References. (PDF 792 kb)
Rights and permissions
About this article
Cite this article
Paeschke, K., Bochman, M., Garcia, P. et al. Pif1 family helicases suppress genome instability at G-quadruplex motifs. Nature 497, 458–462 (2013). https://doi.org/10.1038/nature12149
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12149
This article is cited by
-
In vivo dynamics and regulation of DNA G-quadruplex structures in mammals
Cell & Bioscience (2023)
-
Recognition and coacervation of G-quadruplexes by a multifunctional disordered region in RECQ4 helicase
Nature Communications (2023)
-
Integrative genomic analyses of promoter G-quadruplexes reveal their selective constraint and association with gene activation
Communications Biology (2023)
-
Sublethal effects of acetaminophen exposure on benthic aquatic animal (Hydra magnipapillata)
Molecular & Cellular Toxicology (2023)
-
Ligands stimulating antitumour immunity as the next G-quadruplex challenge
Molecular Cancer (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.