Abstract
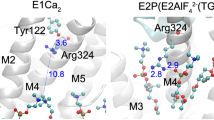
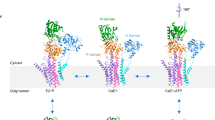
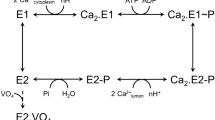
P-type ATPases are ATP-powered ion pumps that establish ion concentration gradients across biological membranes, and are distinct from other ATPases in that the reaction cycle includes an autophosphorylation step. The best studied is Ca2+-ATPase from muscle sarcoplasmic reticulum (SERCA1a), a Ca2+ pump that relaxes muscle cells after contraction, and crystal structures have been determined for most of the reaction intermediates1,2. An important outstanding structure is that of the E1 intermediate, which has empty high-affinity Ca2+-binding sites ready to accept new cytosolic Ca2+. In the absence of Ca2+ and at pH 7 or higher, the ATPase is predominantly in E1, not in E2 (low affinity for Ca2+)3, and if millimolar Mg2+ is present, one Mg2+ is expected to occupy one of the Ca2+-binding sites with a millimolar dissociation constant4,5. This Mg2+ accelerates the reaction cycle4, not permitting phosphorylation without Ca2+ binding. Here we describe the crystal structure of native SERCA1a (from rabbit) in this E1·Mg2+ state at 3.0 Å resolution in addition to crystal structures of SERCA1a in E2 free from exogenous inhibitors, and address the structural basis of the activation signal for phosphoryl transfer. Unexpectedly, sarcolipin6, a small regulatory membrane protein of Ca2+-ATPase7, is bound, stabilizing the E1·Mg2+ state. Sarcolipin is a close homologue of phospholamban, which is a critical mediator of β-adrenergic signal in Ca2+ regulation in heart (for reviews, see, for example, refs 8–10), and seems to play an important role in muscle-based thermogenesis11. We also determined the crystal structure of recombinant SERCA1a devoid of sarcolipin, and describe the structural basis of inhibition by sarcolipin/phospholamban. Thus, the crystal structures reported here fill a gap in the structural elucidation of the reaction cycle and provide a solid basis for understanding the physiological regulation of the calcium pump.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Protein Data Bank
Data deposits
Atomic coordinates and structure factors for the reported crystal structures are deposited in the Protein Data Bank under accession codes 3W5D (E2+Pi), 3W5C (E2), 3W5A (E1•Mg2+ (native enzyme with bound sarcolipin)) and 3W5B (E1•Mg2+ (recombinant enzyme devoid of sarcolipin)).
Change history
08 April 2013
The links for Supplementary Videos 1 and 2 have been updated to work correctly. The direct link for Video 3 is still unavailable. To view this video please use the "download" link in the Supplementary Information section.
References
Toyoshima, C. Structural aspects of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. Arch. Biochem. Biophys. 476, 3–11 (2008)
Møller, J. V., Olesen, C., Winther, A. M. & Nissen, P. The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Q. Rev. Biophys. 43, 501–566 (2010)
Tadini-Buoninsegni, F., Bartolommei, G., Moncelli, M. R., Guidelli, R. & Inesi, G. Pre-steady state electrogenic events of Ca2+/H+ exchange and transport by the Ca2+-ATPase. J. Biol. Chem. 281, 37720–37727 (2006)
Champeil, P., Gingold, M. P., Guillain, F. & Inesi, G. Effect of magnesium on the calcium-dependent transient kinetics of sarcoplasmic reticulum ATPase, studied by stopped flow fluorescence and phosphorylation. J. Biol. Chem. 258, 4453–4458 (1983)
Liu, Y. et al. High-yield heterologous expression of wild type and mutant Ca2+ ATPase: characterization of Ca2+ binding sites by charge transfer. J. Mol. Biol. 391, 858–871 (2009)
Odermatt, A. et al. Characterization of the gene encoding human sarcolipin (SLN), a proteolipid associated with SERCA1: absence of structural mutations in five patients with Brody disease. Genomics 45, 541–553 (1997)
Odermatt, A. et al. Sarcolipin regulates the activity of SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 273, 12360–12369 (1998)
MacLennan, D. H. & Kranias, E. G. Phospholamban: a crucial regulator of cardiac contractility. Nature Rev. Mol. Cell Biol. 4, 566–577 (2003)
Periasamy, M., Bhupathy, P. & Babu, G. J. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc. Res. 77, 265–273 (2008)
Kranias, E. G. & Hajjar, R. J. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ. Res. 110, 1646–1660 (2012)
Bal, N. C. et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nature Med. 18, 1575–1579 (2012)
Obara, K. et al. Structural role of countertransport revealed in Ca2+ pump crystal structure in the absence of Ca2+. Proc. Natl Acad. Sci. USA 102, 14489–14496 (2005)
Jensen, A. M., Sorensen, T. L., Olesen, C., Møller, J. V. & Nissen, P. Modulatory and catalytic modes of ATP binding by the calcium pump. EMBO J. 25, 2305–2314 (2006)
Toyoshima, C. & Nomura, H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418, 605–611 (2002)
Toyoshima, C., Nomura, H. & Tsuda, T. Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature 432, 361–368 (2004)
Lübben, M. et al. Sulfate acts as phosphate analog on the monomeric catalytic fragment of the CPx-ATPase CopB from Sulfolobus solfataricus. J. Mol. Biol. 369, 368–385 (2007)
Toyoshima, C., Yonekura, S., Tsueda, J. & Iwasawa, S. Trinitrophenyl derivatives bind differently from parent adenine nucleotides to Ca2+-ATPase in the absence of Ca2+. Proc. Natl Acad. Sci. USA 108, 1833–1838 (2011)
Holloway, C. E. & Melnik, M. Magnesium compounds: classification and analysis of crystallographic and structural data. J. Organomet. Chem. 465, 1–63 (1994)
Cantilina, T., Sagara, Y., Inesi, G. & Jones, L. R. Comparative studies of cardiac and skeletal sarcoplasmic reticulum ATPases. Effect of a phospholamban antibody on enzyme activation by Ca2+. J. Biol. Chem. 268, 17018–17025 (1993)
Afara, M. R., Trieber, C. A., Ceholski, D. K. & Young, H. S. Peptide inhibitors use two related mechanisms to alter the apparent calcium affinity of the sarcoplasmic reticulum calcium pump. Biochemistry 47, 9522–9530 (2008)
Bhupathy, P., Babu, G. J., Ito, M. & Periasamy, M. Threonine-5 at the N-terminus can modulate sarcolipin function in cardiac myocytes. J. Mol. Cell. Cardiol. 47, 723–729 (2009)
Tupling, A. R. et al. Enhanced Ca2+ transport and muscle relaxation in skeletal muscle from sarcolipin-null mice. Am. J. Physiol. Cell Physiol. 301, C841–C849 (2011)
Asahi, M. et al. Sarcolipin regulates sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) by binding to transmembrane helices alone or in association with phospholamban. Proc. Natl Acad. Sci. USA 100, 5040–5045 (2003)
Toyoshima, C. et al. Modeling of the inhibitory interaction of phospholamban with the Ca2+ ATPase. Proc. Natl Acad. Sci. USA 100, 467–472 (2003)
Chen, Z., Akin, B. L., Stokes, D. L. & Jones, L. R. Cross-linking of C-terminal residues of phospholamban to the Ca2+ pump of cardiac sarcoplasmic reticulum to probe spatial and functional interactions within the transmembrane domain. J. Biol. Chem. 281, 14163–14172 (2006)
Akin, B. L., Chen, Z. & Jones, L. R. Superinhibitory phospholamban mutants compete with Ca2+ for binding to SERCA2a by stabilizing a unique nucleotide-dependent conformational state. J. Biol. Chem. 285, 28540–28552 (2010)
Bidwell, P., Blackwell, D. J., Hou, Z., Zima, A. V. & Robia, S. L. Phospholamban binds with differential affinity to calcium pump conformers. J. Biol. Chem. 286, 35044–35050 (2011)
Buffy, J. J. et al. Defining the intramembrane binding mechanism of sarcolipin to calcium ATPase using solution NMR spectroscopy. J. Mol. Biol. 358, 420–429 (2006)
Olesen, C., Sørensen, T. L., Nielsen, R. C., Møller, J. V. & Nissen, P. Dephosphorylation of the calcium pump coupled to counterion occlusion. Science 306, 2251–2255 (2004)
Hayward, S. Structural principles governing domain motions in proteins. Proteins 36, 425–435 (1999)
Coll, R. J. & Murphy, A. J. Purification of the CaATPase of sarcoplasmic reticulum by affinity chromatography. J. Biol. Chem. 259, 14249–14254 (1984)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Brünger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Murshudov, G. N., Vagin, A. A., Lebedev, A., Wilson, K. S. & Dodson, E. J. Efficient anisotropic refinement of macromolecular structures using FFT. Acta Crystallogr. D 55, 247–255 (1999)
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005)
MacKerell, A. D., Jr et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998)
Klauda, J. B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B 114, 7830–7843 (2010)
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
McDonald, I. K. & Thornton, J. M. Satisfying hydrogen bonding potential in proteins. J. Mol. Biol. 238, 777–793 (1994)
Kraulis, P. J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991)
Babu, G. J., Bhupathy, P., Carnes, C. A., Billman, G. E. & Periasamy, M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J. Mol. Cell. Cardiol. 43, 215–222 (2007)
Ohnoki, S. & Martonosi, A. Purification and characterization of the proteolipid of rabbit sarcoplasmic reticulum. Biochim. Biophys. Acta 626, 170–178 (1980)
Acknowledgements
We thank H. Suzuki and S. Danko (Asahikawa Medical College) for providing us with much unpublished information. Thanks are also due to S. Hasegawa (JASRI) and H. Mimura for data collection at BL41XU of SPring-8, S. Yonekura for refinement of the E2 crystals and Y. Norimatsu for molecular dynamics simulations. We are grateful to D. B. McIntosh for help in improving the manuscript. This work is part of a long-term project (2009B0025) at SPring-8, and was supported by a Specially Promoted Project Grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to C.T.).
Author information
Authors and Affiliations
Contributions
C.T. and G.I. planned and supervised the study; H.O. carried out all the DNA work; H.O., A.H., S.I. and J.T. performed protein preparation and other biochemical work; S.I. and C.T. crystallized the proteins; G.I. established the methods for large-scale production of recombinant proteins and characterized several mutants for clarifying the activation signal; C.T. and H.O. collected diffraction data and determined the structure; C.T., H.O. and J.T. prepared figures; and C.T. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Table 1 and Supplementary Figures 1-14. (PDF 2306 kb)
Rearrangements of transmembrane helices that form SLN binding cavity
SLN appears as a transparent cylinder in E2 and E1•2Ca2+ in the same position as in E1•Mg2+ to show mismatch between SLN and the binding cavity. Animation derived from Figure 3b. When this article was originally published, only the download link for the video worked. The title link has now been updated to work correctly. (MOV 8399 kb)
Alterations of the binding cavity for SLN
Van der Waals surface of the transmembrane region around the SLN binding cavity. Animation derived from Supplementary Figure 14. When this article was originally published, only the download link for the video worked. The title link has now been updated to work correctly. (MOV 3695 kb)
Movements of the A domain in Mg2+ and Ca2+ binding
Aligned with the P domain and viewed approximately perpendicular to the membrane. D351 is the phosphorylation residue. Animation derived from Figure 4a. The direct link to this video is currently out of action. To view this video please use the "download" link (MOV 1320 kb)
Rearrangements of the cytoplasmic domains in the transition from E1•Mg2+ → E1•ATP
Cytoplasmic domains are aligned with the P7 helix and viewed approximately parallel to the membrane. Animation derived from Figure 4b. (MOV 5667 kb)
Rights and permissions
About this article
Cite this article
Toyoshima, C., Iwasawa, S., Ogawa, H. et al. Crystal structures of the calcium pump and sarcolipin in the Mg2+-bound E1 state. Nature 495, 260–264 (2013). https://doi.org/10.1038/nature11899
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11899
This article is cited by
-
Electrostatic interactions between single arginine and phospholipids modulate physiological properties of sarcoplasmic reticulum Ca2+-ATPase
Scientific Reports (2022)
-
Sarcolipin alters SERCA1a interdomain communication by impairing binding of both calcium and ATP
Scientific Reports (2021)
-
The tertiary structure of the human Xkr8–Basigin complex that scrambles phospholipids at plasma membranes
Nature Structural & Molecular Biology (2021)
-
Angle change of the A-domain in a single SERCA1a molecule detected by defocused orientation imaging
Scientific Reports (2021)
-
The orphan solute carrier SLC10A7 is a novel negative regulator of intracellular calcium signaling
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.