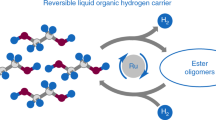
Abstract
Hydrogen produced from renewable resources is a promising potential source of clean energy. With the help of low-temperature proton-exchange membrane fuel cells, molecular hydrogen can be converted efficiently to produce electricity1,2,3,4,5. The implementation of sustainable hydrogen production and subsequent hydrogen conversion to energy is called “hydrogen economy”2. Unfortunately, its physical properties make the transport and handling of hydrogen gas difficult. To overcome this, methanol can be used as a material for the storage of hydrogen, because it is a liquid at room temperature and contains 12.6 per cent hydrogen. However, the state-of-the-art method for the production of hydrogen from methanol (methanol reforming) is conducted at high temperatures (over 200 degrees Celsius) and high pressures (25–50 bar), which limits its potential applications6,7,8. Here we describe an efficient low-temperature aqueous-phase methanol dehydrogenation process, which is facilitated by ruthenium complexes. Hydrogen generation by this method proceeds at 65–95 degrees Celsius and ambient pressure with excellent catalyst turnover frequencies (4,700 per hour) and turnover numbers (exceeding 350,000). This would make the delivery of hydrogen on mobile devices—and hence the use of methanol as a practical hydrogen carrier—feasible.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Stolten, D. (ed.) Hydrogen and Fuel Cells (Wiley-VCH, 2010)
Muradov, N. Z. & Veziroğlu, T. N. “Green” path from fossil-based to hydrogen economy: an overview of carbon-neutral technologies. Int. J. Hydrogen Energy 33, 6804–6839 (2008)
European Commission. European Hydrogen and Fuel Cell Technology Platform “Implementation Plan – Status 2006”http://ec.europa.eu/research/fch/pdf/hfp_ip06_final_20apr2007.pdf (European Technology Platform for Hydrogen and Fuel Cells, European Commission, 2007)
United States Department of Energy. A National Vision of America’s Transition to a Hydrogen Economy — to 2030 and Beyondhttp://hydrogen.energy.gov/pdfs/vision_doc.pdf (United States Department of Energy, 2002)
Pingween, M., Jingguang, L. & Mytelka, L. Hydrogen and Fuel-Cell Activities in China, 2007 http://idl-bnc.idrc.ca/dspace/bitstream/10625/35673/1/127544.pdf 295–308 (United Nations Univ. Press, 2008)
Palo, D. R., Dagle, R. A. & Holladay, J. D. Methanol steam reforming for hydrogen production. Chem. Rev. 107, 3992–4021 (2007)
Cortright, R. D., Davda, R. R. & Dumesic, J. A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 418, 964–967 (2002)
Shabaker, J. W., Davda, R. R., Huber, G. W., Cortright, R. D. & Dumesic, J. A. Aqueous-phase reforming of methanol and ethylene glycol over alumina-supported platinum catalysts. J. Catal. 215, 344–352 (2003)
Florusse, L. J. et al. Stable low-pressure hydrogen clusters stored in a binary clathrate hydrate. Science 306, 469–471 (2004)
Ashcroft, A. T., Cheetham, A. K., Green, M. L. H. & Vernon, P. D. F. Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 352, 225–226 (1991)
Welch, G. C., San Juan, R. R., Masuda, J. D. & Stephan, D. W. Reversible metal-free hydrogen activation. Science 314, 1124–1126 (2006)
Denney, M. C., Pons, V., Hebden, T. J., Heinekey, D. M. & Goldberg, K. I. Efficient catalysis of ammonia borane dehydrogenation. J. Am. Chem. Soc. 128, 12048–12049 (2006)
Reece, S. Y. et al. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 334, 645–648 (2011)
Olah, G., Prakash, G. K. S. & Goeppert, A. Anthropogenic chemical carbon cycle for a sustainable future. J. Am. Chem. Soc. 133, 12881–12898 (2011)
Boddien, A. et al. Efficient dehydrogenation of formic acid using an iron catalyst. Science 333, 1733–1736 (2011)
Nielsen, M. et al. Efficient hydrogen production from alcohols under mild reaction conditions. Angew. Chem. Int. Edn 50, 9593–9597 (2011)
Morton, D. & Cole-Hamilton, D. J. Molecular hydrogen complexes in catalysis: highly efficient hydrogen production from alcoholic substrates catalysed by ruthenium complexes. J. Chem. Soc. Chem. Commun. 17, 1154–1156 (1988)
Nielsen, M., Junge, H., Kammer, A. & Beller, M. Towards a green process for bulk-scale synthesis of ethyl acetate: efficient acceptorless dehydrogenation of ethanol. Angew. Chem. Int. Edn 51, 5711–5713 (2012)
Baratta, W., Bossi, G., Putignano, E. & Rigo, P. Pincer and diamine Ru and Os diphosphane complexes as efficient catalysts for the dehydrogenation of alcohols to ketones. Chemistry 17, 3474–3481 (2011)
Fujita, K., Yoshida, T., Imori, Y. & Yamaguchi, R. Dehydrogenative oxidation of primary and secondary alcohols catalyzed by a Cp*Ir complex having a functional C,N-chelate ligand. Org. Lett. 13, 2278–2281 (2011)
Zhang, J., Leitus, G., Ben-David, Y. & Milstein, D. Facile conversion of alcohols into esters and dihydrogen catalyzed by new ruthenium complexes. J. Am. Chem. Soc. 127, 10840–10841 (2005)
Spasyuk, D., Smith, S. & Gusev, D. G. From esters to alcohols and back with ruthenium and osmium catalysts. Angew. Chem. Int. Edn 51, 2772–2775 (2012)
Kawahara, R., Fujita, K. & &Yamaguchi, R. Dehydrogenative oxidation of alcohols in aqueous media using water-soluble and reusable Cp*Ir catalysts bearing a functional bipyridine ligand. J. Am. Chem. Soc. 134, 3643–3646 (2012)
Maenaka, Y., Suenobu, T. & Fukuzumi, S. Hydrogen evolution from aliphatic alcohols and 1,4-selective hydrogenation of NAD+ catalyzed by a [C,N] and a [C,C] cyclometalated organoiridium complex at room temperature in water. J. Am. Chem. Soc. 134, 9417–9427 (2012)
Gunanathan, C. & Milstein, D. Metal-ligand cooperation by aromatization-dearomatization: a new paradigm in bond activation and “green” catalysis. Acc. Chem. Res. 44, 588–602 (2011)
Gunanathan, C., Ben-David, Y. & Milstein, D. Direct synthesis of amides from alcohols and amines with liberation of H2 . Science 317, 790–792 (2007)
Bertoli, M. et al. Osmium and ruthenium catalysts for dehydrogenation of alcohols. Organometallics 30, 3479–3482 (2011)
Friedrich, A., Drees, M., Schmedt auf der Günne, J. & Schneider, S. Highly stereoselective proton/hydride exchange: assistance of hydrogen bonding for the heterolytic splitting of H2 . J. Am. Chem. Soc. 131, 17552–17553 (2009)
Käß, M., Friedrich, A., Drees, M. & Schneider, S. Ruthenium complexes with cooperative PNP ligands: bifunctional catalysts for the dehydrogenation of ammonia–borane. Angew. Chem. Int. Edn 48, 905–907 (2009)
Baratta, W. et al. Ruthenium(II) terdentate CNN complexes: superlative catalysts for the hydrogen-transfer reduction of ketones by reversible insertion of a carbonyl group into the Ru-H bond. Angew. Chem. Int. Ed. 44, 6214–6219 (2005)
Acknowledgements
M.N. thanks the Alexander von Humboldt Foundation for financial support. We thank the BMBF and the Ministry of Science and Education of Mecklenburg-Western Pommerania for the basic funding of this project.
Author information
Authors and Affiliations
Contributions
M.B., M.N. and H.J. designed the project on methanol dehydrogenation. M.N., E.A., H.J., S.G. and M.B. developed the project. M.N. and E.A. performed the catalytic experiments. E.A., W.B., M.N. and H.-J.D. performed mechanistic and analytic studies. M.N., E.A., H.J., S.G. and M.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Figures 1-19, Supplementary Tables 1-4 and Supplementary References. (PDF 1848 kb)
Rights and permissions
About this article
Cite this article
Nielsen, M., Alberico, E., Baumann, W. et al. Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide. Nature 495, 85–89 (2013). https://doi.org/10.1038/nature11891
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11891
This article is cited by
-
Advances in CO2 circulation hydrogen carriers and catalytic processes
Sustainable Energy Research (2024)
-
Hydrogen Production by Aqueous Phase Reforming of Synthetic Sewage Using Pt/C Catalyst: Effect of Reaction Parameters and Pre-Treatment Strategies
Waste and Biomass Valorization (2024)
-
Methyl formate as a hydrogen energy carrier
Nature Catalysis (2023)
-
High quantum efficiency of hydrogen production from methanol aqueous solution with PtCu–TiO2 photocatalysts
Nature Materials (2023)
-
Effects of ancillary ligands in acceptorless benzyl alcohol dehydrogenation mediated by phosphine-free cobalt complexes
Frontiers of Chemical Science and Engineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.