Abstract
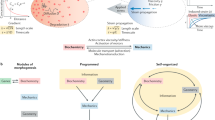
Our knowledge of the principles by which organ architecture develops through complex collective cell behaviours is still limited. Recent work has shown that the shape of such complex tissues as the optic cup forms by self-organization in vitro from a homogeneous population of stem cells. Multicellular self-organization involves three basic processes that are crucial for the emergence of latent intrinsic order. Based on lessons from recent studies, cytosystems dynamics is proposed as a strategy for understanding collective multicellular behaviours, incorporating four-dimensional measurement, theoretical modelling and experimental reconstitution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nieuwkoop, P. D. Inductive interactions in early amphibian development and their general nature. J. Embryol. Exp. Morphol. 89, S333–S347 (1985).
Camazine, S., Deneubourg, J. -L., Franks, N. R., Sneyd, J., Theraulaz, G. & Bonabeau, E. Self-Organization in Biological Systems (Princeton Univ. Press, 2001).
Yates, E. F., Garfinkle, A, Walter, D. O. & Yate, G. B. Self-Organizing Systems: the Emergence of Order (Plenum, 1987).
Chuong, C. M. & Richardson, M. K. Pattern formation today. Int. J. Dev. Biol. 53, 653–658 (2009).
Saetzler, K., Sonnenschein, C. & Soto, A. M. Systems biology beyond networks: generating order from disorder through self-organization. Semin. Cancer Biol. 21, 165–174 (2011).
Dobrescu, R. & Purcarea, V. I. Emergence, self-organization and morphogenesis in biological structures. J. Med. Life 4, 82–90 (2011).
Whitesides, G. M. & Boncheva, M. Beyond molecules: self-assembly of mesoscopic and macroscopic components. Proc. Natl Acad. Sci. USA. 99, 4769–4774 (2002).
Winfree, A. T. Spiral waves of chemical activity. Science 175, 634–636 (1972).
Velarde, M. G. & Normand, C. Convection. Sci. Am. 243, 92–108 (1980).
Forrest, S. B. & Haff, P. K. Mechanics of wind ripple stratigraphy. Science 255, 1240–1243 (1992).
Kauffman, S. A. The Origins of Order: Self-Organization and Selection in Evolution. (Oxford Univ. Press, 1993).
Grassé, P. P. La reconstruction du nid et les coordinations inter-individuelles chez Bellicositermes natalensis et Cubitermes sp. La theorie de la stigmergie: Essai d'interpretation des termites. constructeurs. Insectes Sociaux 6, 41–83 (1959).
Bonabeau, E. Stigmergy. Artif. Life 5, 95–96 (1999).
Whitesides, G. M. & Grzybowski, B. Self-assembly at all scales. Science 295, 2418–2421 (2002).
Caspar, D. L. & Klug, A. Physical principles in the construction of regular viruses. Cold Spring Harb. Symp. Quant. Biol. 27, 1–24 (1962).
Gardner, M. K., Hunt, A. J., Goodson, H. V. & Odde, D. J. Microtubule assembly dynamics: new insights at the nanoscale. Curr. Opin. Cell Biol. 20, 64–70 (2008).
Wilson, H. V. On some phenomena of coalescence and regeneration in sponges. J. Exp. Zool. 5, 245–258 (1907).
Townes, P. L. & Holtfreter, J. Directed movements and selective adhesion of embryonic amphibian cells. J. Exp. Zool. 128, 53–120 (1955).
Dan-Sohkawa, M., Yamanaka, H. & Watanabe, K. Reconstruction of bipinnaria larvae from dissociated embryonic cells of the starfish, Asterina pectinifera. J. Embryol. Exp. Morphol. 94, 47–60 (1986).
Takeichi, M. Self-organization of animal tissues: cadherin-mediated processes. Dev. Cell 21, 24–26 (2011).
Togashi, H. et al. Nectins establish a checkerboard-like cellular pattern in the auditory epithelium. Science 333, 1144–1147 (2011). In this article, the authors demonstrate how differential sets of heterophilic adhesion molecules can form chequerboard-like patterns in the epithelial sheet.
Mori, H., Gjorevski, N., Inman, J. L., Bissell, M. J., Nelson, C. M. Self-organization of engineered epithelial tubules by differential cellular motility. Proc. Natl Acad. Sci. USA. 106, 14890–14895 (2009).
Tanner, K., Mori, H., Mroue, R., Bruni-Cardoso, A. & Bissell, M. J. Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proc. Natl Acad. Sci. USA 109, 1973–1978 (2012).
Sawai, S., Thomason, P. A. & Cox, E. C. An autoregulatory circuit for long-range self-organization in Dictyostelium cell populations. Nature 433, 323–326 (2005).
Thorpe, T. A. History of plant tissue culture. Mol. Biotechnol. 37, 169–180 (2007).
Kondo, S. & Asai, R. A reaction–diffusion wave on the skin of the marine angelfish Pomacanthus. Nature 376, 765–768 (1995). This article is a pioneering paper that suggested Turing-type patterning occurs in stripe formation in the skin of fish.
Jiang, T. X., Jung, H. S., Widelitz, R. B. & Chuong, C. M. Self-organization of periodic patterns by dissociated feather mesenchymal cells and the regulation of size, number and spacing of primordia. Development 126, 4997–5009 (1999).
Wang, Y., Badea, T. & Nathans, J. Order from disorder: self-organization in mammalian hair patterning. Proc. Natl Acad. Sci. USA 103, 19800–19805 (2006).
Sick, S., Reinker, S., Timmer, J. & Schlake, T. WNT and DKK determine hair follicle spacing through a reaction–diffusion mechanism. Science 314, 1447–1450 (2006).
Plikus, M. V. et al. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science 332, 586–589 (2011). In this article the authors report how local interactions between hair follicles contribute to the global pattern of hair regeneration.
Green, J. B., Smith, J. C. & Gerhart, J. C. Slow emergence of a multithreshold response to activin requires cell-contact-dependent sharpening but not prepattern. Development 120, 2271–2278 (1994).
ten Berge, D. et al. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 3, 508–518 (2008).
Marikawa, Y., Tamashiro, D. A., Fujita, T. C. & Alarcon, V. B. Aggregated P19 mouse embryonal carcinoma cells as a simple in vitro model to study the molecular regulations of mesoderm formation and axial elongation morphogenesis. Genesis 47, 103–106 (2009).
Montesano, R., Matsumoto, K., Nakamura, T. & Orci, L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 67, 901–908 (1991).
Wei, C., Larsen, M., Hoffman, M. P. & Yamada, K. M. Self-organization and branching morphogenesis of primary salivary epithelial cells. Tissue Eng. 13, 721–735 (2007).
Nelson, C. M., Vanduijn, M. M., Inman, J. L., Fletcher, D. A. & Bissell, M. J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 314, 298–300 (2006).
Lecaudey, V., Cakan-Akdogan, G., Norton, W. H. & Gilmour, D. Dynamic Fgf signaling couples morphogenesis and migration in the zebrafish lateral line primordium. Development 135, 2695–2705 (2008). This paper describes the mechanism by which FGF signals drive morphogenesis in lateral-line organs during their collective migration.
Friedl, P. & Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nature Rev. Mol. Cell Biol. 10, 445–457 (2009).
Weber, G. F., Bjerke, M. A. & DeSimone, D. W. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev. Cell 22, 104–115 (2012).
Eiraku, E. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011). This paper describes the how the optic cup self-organization mechanism is driven by local rules in ES cell culture.
Nakano, T. et al. Self-formation of optic cups and storable stratified neural retina from human ES cells. Cell Stem Cell 10, 771–785 (2012).
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008). This paper describes spontaneous layer formation in ES-cell-derived cortical tissues.
Mariani, J. et al. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc. Natl Acad. Sci. USA 109, 12770–12775 (2012).
Suga, H. et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature 480, 57–62 (2011).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Sato. T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011).
Spence, J. R., et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011).
Ikeda, E. et al. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl Acad. Sci. USA 106, 13475–13480 (2009).
Nakao, K. et al. The development of a bioengineered organ germ method. Nature Methods 4, 227–230 (2007).
Zheng, Y. et al. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J. Invest. Dermatol. 124, 867–876 (2005).
Toyoshima, K. E. et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nature Commun. 3, 784 (2012).
Halder, G., Callaerts, P. & Gehring, W. J. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science 267, 1788–1792 (1995).
Cohn, M. J., Izpisúa-Belmonte, J. C., Abud, H., Heath, J. K. & Tickle, C. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell 80, 739–746 (1995).
Adler, R. & Canto-Soler, M. V. Molecular mechanisms of optic vesicle development: complexities, ambiguities and controversies. Dev. Biol. 305, 1–13 (2007).
Fuhrmann, S. Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol. 93, 61–84 (2010).
Spemann, H. Über korrelationen in der Entwicklung des Auges. Verh. Anat. Ges. 15, 61–79 (1901).
Hamburger, V. The Heritage of Experimental Embryology (Oxford Univ. Press, 1988).
Li, R. & Bowerman, B. Symmetry Breaking in Biology (Cold Spring Harbor Press 2010).
Turing, A. The chemical basis of morphogenesis. Phil. Trans. R. Soc. Lond. B 237, 37–72 (1952).
Meinhardt, H. & Gierer, A. Pattern formation by local self-activation and lateral inhibition. BioEssays 22, 753–760 (2000).
Kondo, S. & Miura, T. Reaction–diffusion model as a framework for understanding biological pattern formation. Science 329, 1616–1620 (2010).
Economou, A. D. et al. Periodic stripe formation by a Turing mechanism operating at growth zones in the mammalian palate. Nature Genet. 44, 348–351 (2012).
Palmeirim, I., Henrique, D., Ish-Horowicz, D. & Pourquié, O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell 91, 639–648 (1997). This paper demonstrates that vertebrate segmentation occurs in a tissue-autonomous manner that is dependent on its molecular clock.
Palmeirim, I., Dubrulle, J., Henrique, D., Ish-Horowicz, D. & Pourquié, O. Uncoupling segmentation and somitogenesis in the chick presomitic mesoderm. Dev. Genet. 23, 77–85 (1998).
Ferrell, J. E. Jr. Bistability, bifurcations, and Waddington's epigenetic landscape. Curr. Biol. 22, R458–R466 (2012).
Gardner, T. S., Cantor, C. R. & Collins, J. J. Construction of a genetic toggle switch in Escherichia coli. Nature 403, 339–342 (2000).
Fuhrmann, S. Wnt signaling in eye organogenesis. Organogenesis 4, 60–67 (2008).
Yasumoto, K. et al. Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. EMBO J. 21, 2703–2714 (2002).
Liu, W., Lagutin, O., Swindell, E., Jamrich, M. & Oliver, G. Neuroretina specification in mouse embryos requires Six3-mediated suppression of Wnt8b in the anterior neural plate. J. Clin. Invest. 120, 3568–3577 (2010).
Zhao, S. et al. Patterning the optic neuroepithelium by FGF signaling and Ras activation. Development 128, 5051–5060 (2001).
Hoffman, B. D., Grashoff, C. & Schwartz, M. A. Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323 (2011).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Wada, K., Itoga, K., Okano, T., Yonemura, S. & Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development 138, 3907–3914 (2011). References 72 and 73 together demonstrate crucial roles for hippo-related pathways in cellular mechanotransduction, leading to cellular growth and differentiation.
Sansores-Garcia, L. et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 30, 2325–2335 (2011).
Taniguchi, K. et al. Chirality in planar cell shape contributes to left-right asymmetric epithelial morphogenesis. Science 333, 339–341 (2011).
Savin, T. et al. On the growth and form of the gut. Nature 476, 57–62 (2011). This paper is a pioneering report of morphogenetic tissue mechanics, using quantitative measurement of local tissue viscoelasticity.
Martin, A. C., Kaschube, M. & Wieschaus, E. F. Pulsed contractions of an actin–myosin network drive apical constriction. Nature 457, 495–499 (2009).
Rauzi, M., Lenne, P. F. & Lecuit, T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110–1114 (2010).
He, L., Wang, X., Tang, H. L. & Montell, D. J. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nature Cell Biol. 12, 1133–1142 (2010).
Toyama, Y., Peralta, X. G., Wells, A. R., Kiehart, D. P. & Edwards, G. S. Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science 321, 1683–1686 (2008).
Eiraku, M., Adachi, T. & Sasai, Y. Relaxation-expansion model for self-driven retinal morphogenesis. BioEssays 34, 17–25 (2012).
Megason, S. G. & Fraser, S. E. Digitizing life at the level of the cell: high-performance laser-scanning microscopy and image analysis for in toto imaging of development. Mech. Dev. 120, 1407–1420 (2003).
Keller, P. J., Schmidt, A. D., Wittbrodt, J. & Stelzer, E. H. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322, 1065–1069 (2008). This article is a pioneering study of in toto imaging for cell-lineage tracing.
Truong, T. V., Supatto, W., Koos, D. S., Choi, J. M. & Fraser, S. E. Deep and fast live imaging with two-photon scanned light-sheet microscopy. Nature Methods 8, 757–760 (2011).
Tomer, R., Khairy, K., Amat, F. & Keller, P. J. Quantitative high-speed imaging of entire developing embryos with simultaneous multiview light-sheet microscopy. Nature Methods 9, 755–763 (2012).
Krzic, U., Gunther, S., Saunders, T. E., Streichan, S. J. & Hufnagel, L. Multiview light-sheet microscope for rapid in toto imaging. Nature Methods 9, 730–733 (2012).
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nature Methods 2, 932–940 (2005).
Olivier, N. et al. Cell lineage reconstruction of early zebrafish embryos using label-free nonlinear microscopy. Science 329, 967–971 (2010).
Eliceiri, K. W. et al. Biological imaging software tools. Nature Methods 9, 697–710 (2012).
Zhong, Q., Busetto, A. G., Fededa, J. P., Buhmann, J. M. & Gerlich, D. W. Unsupervised modelling of cell morphology dynamics for time-lapse microscopy. Nature Methods 9, 711–713 (2012).
Moore, S. W., Keller, R. E. & Koehl, M. A. The dorsal involuting marginal zone stiffens anisotropically during its convergent extension in the gastrula of Xenopus laevis. Development 121, 3131–3140 (1995).
Davidson, L. A. Embryo mechanics: balancing force production with elastic resistance during morphogenesis. Curr. Top. Dev. Biol. 95, 215–241 (2011).
Krieg, M. et al. Tensile forces govern germ-layer organization in zebrafish. Nature Cell Biol. 10, 429–436 (2008).
Grashoff, C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010).
Meng, F. & Sachs, F. Orientation-based FRET sensor for real-time imaging of cellular forces. J. Cell Sci. 125, 743–750 (2012).
Yonemura, S., Wada, Y., Watanabe, T., Nagafuchi, A. & Shibata, M. α-Catenin as a tension transducer that induces adherens junction development. Nature Cell Biol. 12, 533–542 (2010).
Hannezo, E., Prost, J. & Joanny, J. F. Instabilities of monolayered epithelia: shape and structure of villi and crypts. Phys. Rev. Lett. 107, 078104 (2011).
Karr, J. R. et al. A whole-cell computational model predicts phenotype from genotype. Cell 150, 389–401 (2012).
Wu, Y. I. et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–108 (2009).
Bénazéraf, B. et al. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 466, 248–252 (2010).
Acknowledgements
I would like to thank my collaborators, especially M. Eiraku, N. Takata, K. Muguruma and T. Adachi, for stimulating strategic discussion on multicellular self-organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Additional information
Reprints and permission information is available at www.nature.com/reprints.
Rights and permissions
About this article
Cite this article
Sasai, Y. Cytosystems dynamics in self-organization of tissue architecture. Nature 493, 318–326 (2013). https://doi.org/10.1038/nature11859
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11859
This article is cited by
-
The QBIT Theory: Consciousness and the Maximum Possible Order
Integrative Psychological and Behavioral Science (2024)
-
Extracellular matrix stiffness mediates uterine repair via the Rap1a/ARHGAP35/RhoA/F-actin/YAP axis
Cell Communication and Signaling (2023)
-
Higher throughput workflow with sensitive, reliable and automatic quantification of myelination in vitro suitable for drug screening
Scientific Reports (2023)
-
Phase intensity nanoscope (PINE) opens long-time investigation windows of living matter
Nature Communications (2023)
-
Predicting cancer stages from tissue energy dissipation
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.