Abstract
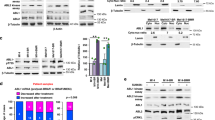
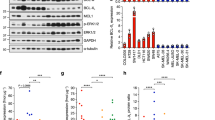
Mutational activation of BRAF is the most prevalent genetic alteration in human melanoma, with ≥50% of tumours expressing the BRAF(V600E) oncoprotein1,2. Moreover, the marked tumour regression and improved survival of late-stage BRAF-mutated melanoma patients in response to treatment with vemurafenib demonstrates the essential role of oncogenic BRAF in melanoma maintenance3,4. However, as most patients relapse with lethal drug-resistant disease, understanding and preventing mechanism(s) of resistance is critical to providing improved therapy5. Here we investigate the cause and consequences of vemurafenib resistance using two independently derived primary human melanoma xenograft models in which drug resistance is selected by continuous vemurafenib administration. In one of these models, resistant tumours show continued dependency on BRAF(V600E)→MEK→ERK signalling owing to elevated BRAF(V600E) expression. Most importantly, we demonstrate that vemurafenib-resistant melanomas become drug dependent for their continued proliferation, such that cessation of drug administration leads to regression of established drug-resistant tumours. We further demonstrate that a discontinuous dosing strategy, which exploits the fitness disadvantage displayed by drug-resistant cells in the absence of the drug, forestalls the onset of lethal drug-resistant disease. These data highlight the concept that drug-resistant cells may also display drug dependency, such that altered dosing may prevent the emergence of lethal drug resistance. Such observations may contribute to sustaining the durability of the vemurafenib response with the ultimate goal of curative therapy for the subset of melanoma patients with BRAF mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
13 February 2013
The y axis label in Fig. 3c was corrected.
References
Fecher, L. A., Amaravadi, R. K. & Flaherty, K. T. The MAPK pathway in melanoma. Curr. Opin. Oncol. 20, 183–189 (2008)
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002)
Sosman, J. A. et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 366, 707–714 (2012)
Bollag, G. et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467, 596–599 (2010)
Flaherty, K. T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010)
Nazarian, R. et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 468, 973–977 (2010)
Wagle, N. et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J. Clin. Oncol. 29, 3085–3096 (2011)
Poulikakos, P. I. et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 480, 387–390 (2011)
Shi, H. et al. Melanoma whole-exome sequencing identifies V600EB-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nature Commun. 3, 724 (2012)
Bennett, D. C. How to make a melanoma: what do we know of the primary clonal events? Pigment Cell Melanoma Res. 21, 27–38 (2008)
Petti, C. et al. Coexpression of NRASQ61R and BRAFV600E in human melanoma cells activates senescence and increases susceptibility to cell-mediated cytotoxicity. Cancer Res. 66, 6503–6511 (2006)
Zhu, J., Woods, D., McMahon, M. & Bishop, J. M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12, 2997–3007 (1998)
Woods, D. et al. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol. Cell. Biol. 17, 5598–5611 (1997)
Marshall, C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal regulated kinase activation. Cell 80, 179–185 (1995)
Johannessen, C. M. et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 468, 968–972 (2010)
Villanueva, J. et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 18, 683–695 (2010)
Tap, W. D. et al. Pharmacodynamic characterization of the efficacy signals due to selective BRAF inhibition with PLX4032 in malignant melanoma. Neoplasia 12, 637–649 (2010)
Hoeflich, K. P. et al. Oncogenic BRAF is required for tumor growth and maintenance in melanoma models. Cancer Res. 66, 999–1006 (2006)
Hingorani, S. R., Jacobetz, M. A., Robertson, G. P., Herlyn, M. & Tuveson, D. A. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 63, 5198–5202 (2003)
Neyns, B., Seghers, A. C., Wilgenhof, S. & Lebbe, C. Successful rechallenge in two patients with BRAF-V600-mutant melanoma who experienced previous progression during treatment with a selective BRAF inhibitor. Melanoma Res. 22, 466–472 (2012)
Greaves, M. & Maley, C. C. Clonal evolution in cancer. Nature 481, 306–313 (2012)
Chmielecki, J. et al. EGFR-mutant lung adenocarcinomas treated first-line with the novel EGFR inhibitor, XL647, can subsequently retain moderate sensitivity to erlotinib. J. Thorac. Oncol. 7, 434–442 (2012)
Acknowledgements
We thank the members of the Novartis Institutes for BioMedical Reseach (NIBR) Pharmacology department for technical support, comments and discussions during the course of this work. We thank C. Voliva, N. Aziz and E. Collisson for discussions. We thank B. Weisburd and the rest of the NIBR Bioinformatics department for assistance with exome sequencing data analysis. We thank S. Kaufman for sharing her knowledge of cell-based assays. We thank V. Marsh, N. Rosen, P. Poulikakos and D. Solit for providing additional advice and reagents. M.D.T. was supported by an NIBR Presidential Postdoctoral Fellowship. M.M. acknowledges support from the Melanoma Research Alliance and the National Cancer Institute (R01-CA176839). A.S.L. was supported by a National Research Service Award T32 training grant HL007185.
Author information
Authors and Affiliations
Contributions
M.D.T., M.M. and D.D.S. designed all experiments. M.D.T. performed in vivo and in vitro experiments and collected data. M.D.T., M.M. and D.D.S. analysed data, wrote the paper and guided the manuscript through review. F.S. assisted in performing in vivo experiments. A.S.L. carried out the clonogenic assay with the SK-Mel-239-C3 cells. M.P.L. and R.D. provided the human patient biopsy samples, and R.D. assisted with data analysis and interpretation. W.R.S. and N.K.P. provided input on the experimental approach and on the manuscript. M.M. and D.D.S. are co-senior authors of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-6 and Supplementary Table 1. (PDF 416 kb)
Rights and permissions
About this article
Cite this article
Das Thakur, M., Salangsang, F., Landman, A. et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 494, 251–255 (2013). https://doi.org/10.1038/nature11814
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11814
This article is cited by
-
BRAF — a tumour-agnostic drug target with lineage-specific dependencies
Nature Reviews Clinical Oncology (2024)
-
Polysaccharide of Alocasia cucullata Exerts Antitumor Effect by Regulating Bcl-2, Caspase-3 and ERK1/2 Expressions during Long-Time Administration
Chinese Journal of Integrative Medicine (2024)
-
DNA replication stress and mitotic catastrophe mediate sotorasib addiction in KRASG12C-mutant cancer
Journal of Biomedical Science (2023)
-
Drug addiction unveils a repressive methylation ceiling in EZH2-mutant lymphoma
Nature Chemical Biology (2023)
-
Systematic profiling of conditional pathway activation identifies context-dependent synthetic lethalities
Nature Genetics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.