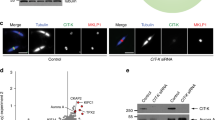
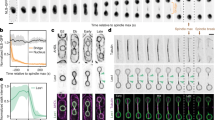
Abstract
At the end of cell division, cytokinesis splits the cytoplasm of nascent daughter cells and partitions segregated sister genomes1,2. To coordinate cell division with chromosome segregation, the mitotic spindle controls cytokinetic events at the cell envelope. The spindle midzone stimulates the actomyosin-driven contraction of the cleavage furrow, which proceeds until the formation of a microtubule-rich intercellular bridge with the midbody at its centre. The midbody directs the final membrane abscission reaction1,2 and has been proposed to attach the cleavage furrow to the intercellular bridge3. How the mitotic spindle is connected to the plasma membrane during cytokinesis is not understood. Here we identify a plasma membrane tethering activity in the centralspindlin protein complex, a conserved component of the spindle midzone and midbody4. We demonstrate that the C1 domain of the centralspindlin subunit MgcRacGAP associates with the plasma membrane by interacting with polyanionic phosphoinositide lipids. Using X-ray crystallography we determine the structure of this atypical C1 domain. Mutations in the hydrophobic cap and in basic residues of the C1 domain of MgcRacGAP prevent association of the protein with the plasma membrane, and abrogate cytokinesis in human and chicken cells. Artificial membrane tethering of centralspindlin restores cell division in the absence of the C1 domain of MgcRacGAP. Although C1 domain function is dispensable for the formation of the midzone and midbody, it promotes contractility and is required for the attachment of the plasma membrane to the midbody, a long-postulated function of this organelle3. Our analysis suggests that centralspindlin links the mitotic spindle to the plasma membrane to secure the final cut during cytokinesis in animal cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Protein Data Bank
Data deposits
Coordinates and structure factors for the C1domain of MgcRacGAP have been deposited at the Protein Data Bank (http://www.rcsb.org/pdb) under accession code 4B6D.
References
Fededa, J. P. & Gerlich, D. W. Molecular control of animal cell cytokinesis. Nature Cell Biol. 14, 440–447 (2012)
Green, R. A., Paluch, E. & Oegema, K. Cytokinesis in animal cells. Annu. Rev. Cell Dev. Biol. 28, 29–58 (2012)
Mullins, J. M. & Biesele, J. J. Terminal phase of cytokinesis in D-98s cells. J. Cell Biol. 73, 672–684 (1977)
Glotzer, M. The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nature Rev. Mol. Cell Biol. 10, 9–20 (2009)
Mishima, M., Kaitna, S. & Glotzer, M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell 2, 41–54 (2002)
Pavicic-Kaltenbrunner, V., Mishima, M. & Glotzer, M. Cooperative assembly of CYK-4/MgcRacGAP and ZEN-4/MKLP1 to form the centralspindlin complex. Mol. Biol. Cell 18, 4992–5003 (2007)
Bastos, R. N., Penate, X., Bates, M., Hammond, D. & Barr, F. A. CYK4 inhibits Rac1-dependent PAK1 and ARHGEF7 effector pathways during cytokinesis. J. Cell Biol. 198, 865–880 (2012)
Canman, J. C. et al. Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science 322, 1543–1546 (2008)
Loria, A., Longhini, K. M. & Glotzer, M. The RhoGAP domain of CYK-4 has an essential role in RhoA activation. Curr. Biol. 22, 213–219 (2012)
Miller, A. L. & Bement, W. M. Regulation of cytokinesis by Rho GTPase flux. Nature Cell Biol. 11, 71–77 (2009)
Carlton, J. G. & Martin-Serrano, J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science 316, 1908–1912 (2007)
Simon, G. C. et al. Sequential Cyk-4 binding to ECT2 and FIP3 regulates cleavage furrow ingression and abscission during cytokinesis. EMBO J. 27, 1791–1803 (2008)
Somers, W. G. & Saint, R. A. RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell 4, 29–39 (2003)
Su, K. C., Takaki, T. & Petronczki, M. Targeting of the RhoGEF Ect2 to the equatorial membrane controls cleavage furrow formation during cytokinesis. Dev. Cell 21, 1104–1115 (2011)
Yüce, O., Piekny, A. & Glotzer, M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol. 170, 571–582 (2005)
Zhao, W. M., Seki, A. & Fang, G. Cep55, a microtubule-bundling protein, associates with centralspindlin to control the midbody integrity and cell abscission during cytokinesis. Mol. Biol. Cell 17, 3881–3896 (2006)
Colón-González, F. & Kazanietz, M. G. C1 domains exposed: from diacylglycerol binding to protein–protein interactions. Biochim. Biophys. Acta 1761, 827–837 (2006)
Zhang, G., Kazanietz, M. G., Blumberg, P. M. & Hurley, J. H. Crystal structure of the cys2 activator-binding domain of protein kinase C delta in complex with phorbol ester. Cell 81, 917–924 (1995)
Hutterer, A., Glotzer, M. & Mishima, M. Clustering of centralspindlin is essential for its accumulation to the central spindle and the midbody. Curr. Biol. 19, 2043–2049 (2009)
Hammond, G. R. et al. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science 337, 727–730 (2012)
Lomize, M. A., Pogozheva, I. D., Joo, H., Mosberg, H. I. & Lomize, A. L. OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res. 40, D370–D376 (2012)
Heo, W. D. et al. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314, 1458–1461 (2006)
Zavortink, M., Contreras, N., Addy, T., Bejsovec, A. & Saint, R. Tum/RacGAP50C provides a critical link between anaphase microtubules and the assembly of the contractile ring in Drosophila melanogaster. J. Cell Sci. 118, 5381–5392 (2005)
Yamada, T., Hikida, M. & Kurosaki, T. Regulation of cytokinesis by mgcRacGAP in B lymphocytes is independent of GAP activity. Exp. Cell Res. 312, 3517–3525 (2006)
Echard, A., Hickson, G. R., Foley, E. & O'Farrell, P. H. Terminal cytokinesis events uncovered after an RNAi screen. Curr. Biol. 14, 1685–1693 (2004)
Dambournet, D. et al. Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nature Cell Biol. 13, 981–988 (2011)
Hu, C. K., Coughlin, M. & Mitchison, T. J. Midbody assembly and its regulation during cytokinesis. Mol. Biol. Cell 23, 1024–1034 (2012)
Kechad, A., Jananji, S., Ruella, Y. & Hickson, G. R. Anillin acts as a bifunctional linker coordinating midbody ring biogenesis during cytokinesis. Curr. Biol. 22, 197–203 (2012)
Fujiwara, T. et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437, 1043–1047 (2005)
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010)
Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D 62, 72–82 (2006)
Padilla, J. E. & Yeates, T. O. A statistic for local intensity differences: robustness to anisotropy and pseudo-centering and utility for detecting twinning. Acta Crystallogr. D 59, 1124–1130 (2003)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010)
Sheldrick, G. M. Experimental phasing with SHELXC/D/E: combining chain tracing with density modification. Acta Crystallogr. D 66, 479–485 (2010)
Brodersen, D. E. et al. Applications of single-wavelength anomalous dispersion at high and atomic resolution. Acta Crystallogr. D 56, 431–441 (2000)
Abrahams, J. P. & Leslie, A. G. Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta Crystallogr. D 52, 30–42 (1996)
Vagin, A. & Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. D 66, 22–25 (2010)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Steigemann, P. et al. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell 136, 473–484 (2009)
Burkel, B. M., von Dassow, G. & Bement, W. M. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil. Cytoskeleton 64, 822–832 (2007)
Petronczki, M., Glotzer, M., Kraut, N. & Peters, J. M. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev. Cell 12, 713–725 (2007)
Lénárt, P. et al. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol. 17, 304–315 (2007)
Piekny, A. J. & Glotzer, M. Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr. Biol. 18, 30–36 (2008)
Arakawa, H., Lodygin, D. & Buerstedde, J. M. Mutant loxP vectors for selectable marker recycle and conditional knock-outs. BMC Biotechnol. 1, 7 (2001)
Acknowledgements
We would like to thank W. Bement, W. Earnshaw, J. Gannon, D. Gerlich, M. Glotzer, G. Hammond, M. Hikida, H. Hochegger, R. Irvine, M. Kurosaki, B. Larijani, P. Parker, A. Piekny, R. Prekeris, E. Sahai, K. Samejima and M. Symons for reagents and advice. Work in the Petronczki laboratory is supported by Cancer Research UK and the EMBO Young Investigator Programme. S.L. acknowledges support from an EMBO Long-Term Fellowship.
Author information
Authors and Affiliations
Contributions
M.P. and S.L. designed the experiments. S.L. conducted all experiments with the following exceptions. The imaging data shown in Fig. 4a were recorded by K.-C. S. The structure of the C1 domain was determined by V.E.P. and P.C. Electron microscopy analysis was carried out with contributions from K.B. and L.M.C. The analysis of MgcRacGAP-R385A was carried out by S.S. Lipid interaction assays were carried out by T.T. and N.D. The manuscript was written by M.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9, Supplementary References and Supplementary Table 1, which contains data collection, phasing and refinement statistics for the structure determination of MgcRacGAP’s C1 domain by X-ray crystallography. (PDF 9142 kb)
The C1 domain of MgcRacGAP is atypical (related to Figure 2)
This video shows confocal time-lapse series of HeLa Kyoto cells co-expressing mCherry–H2B (red) and a FLAc-tagged version of the C1B domain of human PKCα (white) (left panel) or a FLAc-tagged version of the C1 domain of human MgcRacGAP (white) (right panel). Frames were acquired every 30 seconds. 1 μM TPA was added at t = 0 sec. (MOV 1855 kb)
Live-cell imaging of the plasma membrane and MgcRacGAP dynamics during cytokinesis (related to Figure 4)
This video shows confocal time-lapse series of HeLa Kyoto cell stably co-expressing MyrPalm–mCherry (red) and the indicated MgcRacGAPr–FLAc transgenes (white). Cells were recorded 28 to 48 h after transfection with MgcRacGAP siRNA. Frames were acquired every 2 min. Time point t = 0 min was set to the metaphase-toanaphase transition. (MOV 8773 kb)
Rights and permissions
About this article
Cite this article
Lekomtsev, S., Su, KC., Pye, V. et al. Centralspindlin links the mitotic spindle to the plasma membrane during cytokinesis. Nature 492, 276–279 (2012). https://doi.org/10.1038/nature11773
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11773
This article is cited by
-
The Flemmingsome reveals an ESCRT-to-membrane coupling via ALIX/syntenin/syndecan-4 required for completion of cytokinesis
Nature Communications (2020)
-
Membrane and organelle dynamics during cell division
Nature Reviews Molecular Cell Biology (2020)
-
MED12 exerts an emerging role in actin-mediated cytokinesis via LIMK2/cofilin pathway in NSCLC
Molecular Cancer (2019)
-
Building bridges between chromosomes: novel insights into the abscission checkpoint
Cellular and Molecular Life Sciences (2019)
-
Actomyosin drives cancer cell nuclear dysmorphia and threatens genome stability
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.