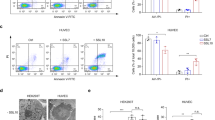
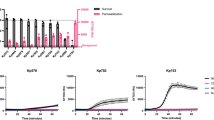
Abstract
Pore-forming toxins are critical virulence factors for many bacterial pathogens and are central to Staphylococcus aureus-mediated killing of host cells. S. aureus encodes pore-forming bi-component leukotoxins that are toxic towards neutrophils, but also specifically target other immune cells. Despite decades since the first description of staphylococcal leukocidal activity, the host factors responsible for the selectivity of leukotoxins towards different immune cells remain unknown. Here we identify the human immunodeficiency virus (HIV) co-receptor CCR5 as a cellular determinant required for cytotoxic targeting of subsets of myeloid cells and T lymphocytes by the S. aureus leukotoxin ED (LukED). We further demonstrate that LukED-dependent cell killing is blocked by CCR5 receptor antagonists, including the HIV drug maraviroc. Remarkably, CCR5-deficient mice are largely resistant to lethal S. aureus infection, highlighting the importance of CCR5 targeting in S. aureus pathogenesis. Thus, depletion of CCR5+ leukocytes by LukED suggests a new immune evasion mechanism of S. aureus that can be therapeutically targeted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Foster, T. J. Immune evasion by staphylococci. Nature Rev. Microbiol. 3, 948–958 (2005)
Bischofberger, M., Iacovache, I. & Gisou van der Goot, F. Pathogenic pore-forming proteins: function and host response. Cell Host Microbe 12, 266–275 (2012)
Menestrina, G. et al. Ion channels and bacterial infection: the case of β-barrel pore-forming protein toxins of Staphylococcus aureus . FEBS Lett. 552, 54–60 (2003)
Dumont, A. L. et al. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol. Microbiol. 79, 814–825 (2011)
Van de Velde, H. Etude sur le mécanisme de la virulence du staphylocoque pyogène. Cellule 10, 403–460 (1894)
Panton, P. N. & Valentine, F. C. O. Staphylococcal toxin. Lancet i, 506–508 (1932)
Alonzo, F., III et al. Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo . Mol. Microbiol. 83, 423–435 (2012)
Morner, A. et al. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 73, 2343–2349 (1999)
Deng, H. et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381, 661–666 (1996)
Doranz, B. J. et al. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85, 1149–1158 (1996)
Didigu, C. A. & Doms, R. W. Novel approaches to inhibit HIV entry. Viruses 4, 309–324 (2012)
Strizki, J. M. et al. Discovery and characterization of vicriviroc (SCH 417690), a CCR5 antagonist with potent activity against human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 49, 4911–4919 (2005)
Baba, M. et al. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl Acad. Sci. USA 96, 5698–5703 (1999)
Lee, B. et al. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J. Biol. Chem. 274, 9617–9626 (1999)
Rich, R. L., Miles, A. R., Gale, B. K. & Myszka, D. G. Detergent screening of a G-protein-coupled receptor using serial and array biosensor technologies. Anal. Biochem. 386, 98–104 (2009)
Liu, R. et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86, 367–377 (1996)
Samson, M. et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382, 722–725 (1996)
El Hed, A. et al. Susceptibility of human Th17 cells to human immunodeficiency virus and their perturbation during infection. J. Infect. Dis. 201, 843–854 (2010)
Wan, Q. et al. Cytokine signals through PI-3 kinase pathway modulate Th17 cytokine production by CCR6+ human memory T cells. J. Exp. Med. 208, 1875–1887 (2011)
Saita, Y., Kondo, M. & Shimizu, Y. Species selectivity of small-molecular antagonists for the CCR5 chemokine receptor. Int. Immunopharmacol. 7, 1528–1534 (2007)
Golding, H. et al. Inhibition of HIV-1 infection by a CCR5-binding cyclophilin from Toxoplasma gondii . Blood 102, 3280–3286 (2003)
Aliberti, J. et al. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat. Immunol. 4, 485–490 (2003)
Rahbar, R., Murooka, T. T. & Fish, E. N. Role for CCR5 in dissemination of vaccinia virus in vivo . J. Virol. 83, 2226–2236 (2009)
Lalani, A. S. et al. Use of chemokine receptors by poxviruses. Science 286, 1968–1971 (1999)
Hummel, S., Schmidt, D., Kremeyer, B., Herrmann, B. & Oppermann, M. Detection of the CCR5- Δ 32 HIV resistance gene in Bronze Age skeletons. Genes Immun. 6, 371–374 (2005)
Lucotte, G. Frequencies of 32 base pair deletion of the (Δ32) allele of the CCR5 HIV-1 co-receptor gene in Caucasians: a comparative analysis. Infect. Genet. Evol. 1, 201–205 (2002)
Hedrick, P. W. & Verrelli, B. C. ‘Ground truth’ for selection on CCR5-Δ32 . Trends Genet. 22, 293–296 (2006)
Moore, P. C. & Lindsay, J. A. Molecular characterisation of the dominant UK methicillin-resistant Staphylococcus aureus strains, EMRSA-15 and EMRSA-16. J. Med. Microbiol. 51, 516–521 (2002)
Vandenesch, F. et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9, 978–984 (2003)
von Eiff, C., Friedrich, A. W., Peters, G. & Becker, K. Prevalence of genes encoding for members of the staphylococcal leukotoxin family among clinical isolates of Staphylococcus aureus . Diagn. Microbiol. Infect. Dis. 49, 157–162 (2004)
DeLeo, F. R. et al. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus . Proc Natl Acad Sci USA 108, 18091–18096 (2011)
Zielinski, C. E. et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484, 514–518 (2012)
Cho, J. S. et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120, 1762–1773 (2010)
Lin, L. et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 5, e1000703 (2009)
Oswald-Richter, K. et al. Identification of a CCR5-expressing T cell subset that is resistant to R5-tropic HIV infection. PLoS Pathog. 3, e58 (2007)
Gramberg, T., Sunseri, N. & Landau, N. R. Evidence for an activation domain at the amino terminus of simian immunodeficiency virus Vpx. J. Virol. 84, 1387–1396 (2010)
Manel, N. et al. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature 467, 214–217 (2010)
Berro, R. et al. Multiple CCR5 conformations on the cell surface are used differentially by human immunodeficiency viruses resistant or sensitive to CCR5 inhibitors. J. Virol. 85, 8227–8240 (2011)
Stenlund, P., Babcock, G. J., Sodroski, J. & Myszka, D. G. Capture and reconstitution of G protein-coupled receptors on a biosensor surface. Anal. Biochem. 316, 243–250 (2003)
Navratilova, I., Sodroski, J. & Myszka, D. G. Solubilization, stabilization, and purification of chemokine receptors using biosensor technology. Anal. Biochem. 339, 271–281 (2005)
Navratilova, I., Dioszegi, M. & Myszka, D. G. Analyzing ligand and small molecule binding activity of solubilized GPCRs using biosensor technology. Anal. Biochem. 355, 132–139 (2006)
Navratilova, I., Pancera, M., Wyatt, R. T. & Myszka, D. G. A biosensor-based approach toward purification and crystallization of G protein-coupled receptors. Anal. Biochem. 353, 278–283 (2006)
Caccuri, F. et al. HIV-1 matrix protein p17 promotes angiogenesis via chemokine receptors CXCR1 and CXCR2. Proc. Natl Acad. Sci. USA 109, 14580–14585 (2012)
Giagulli, C. et al. HIV-1 matrix protein p17 binds to the IL-8 receptor CXCR1 and shows IL-8-like chemokine activity on monocytes through Rho/ROCK activation. Blood 119, 2274–2283 (2012)
Acknowledgements
We thank members of the Torres laboratory, D. R. Littman, M. Lu, and A. Darwin for reading this manuscript. We also thank V. KewalRamani for providing reagents, and S. Polsky for assistance with purification of PBMCs. This research was supported by New York University School of Medicine Development Funds, an American Heart Association Scientist Development Grant (09SDG2060036) to V.J.T. and National Institutes of Health (NIH) grants R56-AI091856-01A1 to V.J.T., NIH training grant T32-AI007180 to F.A., A.L.D. and S.A.R., NIH R42-MH084372-02A1 to D.G.M., and NIH R21-AI087973 and R01-AI065303 grants to D.U.
Author information
Authors and Affiliations
Contributions
F.A. and V.J.T. identified CCR5 as the LukED receptor. F.A., A.L.D. and T.R.-R. purified the toxins. S.A.R. generated the CCR5 shRNA knockdown and CCR5 over-expressing cells. F.A., S.A.R. and A.L.D. performed the cytotoxicity assays of cell lines. L.K. purified and sorted primary cells. D.U. designed the experiments for the effect of LukED on human cells. L.K. performed the experiments with primary human cells and S.A.R. performed the HIV infection experiments. F.A. and T.R.-R. conducted the biochemical and cell binding studies with LukED and GFP fusion proteins. F.A. and T.R.-R. conducted the animal studies. D.M. performed the surface plasmon resonance experiments. N.R.L. provided cDNA plasmids and the Δ32 CCR5 primary cells. V.J.T. and D.U. coordinated and directed the project. All authors discussed the data and commented on the manuscript. F.A., D.U. and V.J.T. interpreted the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-13. (PDF 1336 kb)
Rights and permissions
About this article
Cite this article
Alonzo III, F., Kozhaya, L., Rawlings, S. et al. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 493, 51–55 (2013). https://doi.org/10.1038/nature11724
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11724
This article is cited by
-
The chemokine receptor CCR5: multi-faceted hook for HIV-1
Retrovirology (2024)
-
Story of Pore-Forming Proteins from Deadly Disease-Causing Agents to Modern Applications with Evolutionary Significance
Molecular Biotechnology (2023)
-
Genetic variation of staphylococcal LukAB toxin determines receptor tropism
Nature Microbiology (2021)
-
Decoy exosomes provide protection against bacterial toxins
Nature (2020)
-
Neutrophil chemoattractant receptors in health and disease: double-edged swords
Cellular & Molecular Immunology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.