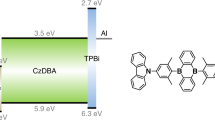
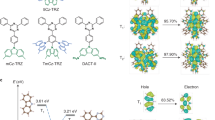
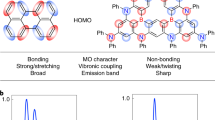
Abstract
The inherent flexibility afforded by molecular design has accelerated the development of a wide variety of organic semiconductors over the past two decades. In particular, great advances have been made in the development of materials for organic light-emitting diodes (OLEDs), from early devices based on fluorescent molecules1 to those using phosphorescent molecules2,3. In OLEDs, electrically injected charge carriers recombine to form singlet and triplet excitons in a 1:3 ratio1; the use of phosphorescent metal–organic complexes exploits the normally non-radiative triplet excitons and so enhances the overall electroluminescence efficiency2,3. Here we report a class of metal-free organic electroluminescent molecules in which the energy gap between the singlet and triplet excited states is minimized by design4, thereby promoting highly efficient spin up-conversion from non-radiative triplet states to radiative singlet states while maintaining high radiative decay rates, of more than 106 decays per second. In other words, these molecules harness both singlet and triplet excitons for light emission through fluorescence decay channels, leading to an intrinsic fluorescence efficiency in excess of 90 per cent and a very high external electroluminescence efficiency, of more than 19 per cent, which is comparable to that achieved in high-efficiency phosphorescence-based OLEDs3.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pope, M., Kallmann, H. P. & Magnante, P. Electroluminescence in organic crystals. J. Chem. Phys. 38, 2042–2043 (1963)
Baldo, M. A., Lamansky, S., Burrows, P. E., Thompson, M. E. & Forrest, S. R. Very high-efficiency green organic light-emitting devices based on electrophosphorescence. Appl. Phys. Lett. 75, 4–6 (1999)
Adachi, C., Baldo, M. A., Thompson, M. E. & Forrest, S. R. Nearly 100% internal phosphorescence efficiency in an organic light emitting device. J. Appl. Phys. 90, 5048–5051 (2001)
Endo, A. et al. Efficient up-conversion of triplet excitons into a singlet state and its application to organic light emitting diodes. Appl. Phys. Lett. 98, 083302 (2011)
Bernanose, A., Comte, M. & Vouaux, P. A new method of emission of light by certain organic compounds. J. Chim. Phys. 50, 64–68 (1953)
Tsutsui, T. & Saito, S. Organic Multilayer-Dye Electroluminescent Diodes: Is There Any Difference with Polymer LED? 127–131 (Kluwer, 1993)
Rothberg, L. J. & Lovinger, A. J. Status of and prospects for organic electroluminescence. J. Mater. Res. 11, 3174–3187 (1996)
Wilson, J. S. et al. Spin-dependent exciton formation in π-conjugated compounds. Nature 413, 828–831 (2001)
Segal, M., Baldo, M. A., Holmes, R. J., Forrest, S. R. & Soos, Z. G. Excitonic singlet-triplet ratios in molecular and polymeric organic materials. Phys. Rev. B 68, 075211 (2003)
Cao, Y., Parker, I. D., Yu, G., Zhang, C. & Heeger, A. J. Improved quantum efficiency for electroluminescence in semiconducting polymers. Nature 397, 414–417 (1999)
Wohlgenannt, M., Jiang, X. M., Vardeny, Z. V. & Janssen, R. A. J. Conjugation-length dependence of spin-dependant exciton formation rates in p-conjugated oligomers and polymers. Phys. Rev. Lett. 88, 197401 (2002)
Shuai, Z., Beljonne, D., Silbey, R. J. & Brédas, L. J. Singlet and triplet exciton formation rates in conjugated polymer light-emitting diodes. Phys. Rev. Lett. 84, 131–134 (2000)
Tsutsui, T., Adachi, C. & Saito, S. in Photochemical Processes in Organized Molecular Systems (ed. Honda, K.) 437–450 (Elsevier, 1991)
Kido, J., Nagai, K. & Ohashi, Y. Electroluminescence in a terbium complex. Chem. Lett. 19, 657–660 (1990)
Endo, A. et al. Thermally activated delayed fluorescence from Sn4+–porphyrin complexes and their application to organic light-emitting diodes: a novel mechanism for electroluminescence. Adv. Mater. 21, 4802–4806 (2009)
Akai, N., Kudoh, S. & Nakata, M. Lowest excited triplet states of 1,2- and 1,4-dicyanobenzenes by low-temperature matrix-isolation infrared spectroscopy and density-functional-theory calculation. Chem. Phys. Lett. 371, 655–661 (2003)
Wakamiya, A., Mori, K. & Yamaguchi, S. 3-boryl-2,2′-bithiophene as a versatile core skeleton for full-color highly emissive organic solids. Angew. Chem. Int. Ed. 46, 4273–4276 (2007)
Hariharan, P. C. & Pople, J. A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 28, 213–222 (1973)
Frisch, M. J. et al. Gaussian 09, Revision C. 01http://www.gaussian.com/index.htm (2010)
Zhao, Y. & Truhlar, D. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008)
Improta, R., Barone, V., Scalmani, G. & Frisch, M. J. A state-specific polarizable continuum model time dependent density functional method for excited state calculations in solution. J. Chem. Phys. 125, 054103 (2006)
Improta, R., Scalmani, G., Frisch, M. J. & Barone, V. Toward effective and reliable fluorescence energies in solution by a new state specific polarizable continuum model time dependent density functional theory approach. J. Chem. Phys. 127, 074504 (2007)
Martin, R. L. Natural transition orbitals. J. Chem. Phys. 118, 4775–4777 (2003)
Goushi, K., Yoshida, K., Sato, K. & Adachi, C. Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion. Nature Photon. 6, 253–258 (2012)
Smith, L. H., Wasey, J. A. E. & Barnes, W. L. Light outcoupling efficiency of top-emitting organic light-emitting diodes. Appl. Phys. Lett. 84, 2986–2988 (2004)
Tanaka, D. et al. Ultra high efficiency green organic light-emitting devices. Jpn. J. Appl. Phys. 46, L10–L12 (2007)
Turro, N. J. Modern Molecular Photochemistry 98–100 (Benjamin Cummings, 1978)
Beljonne, D., Shuai, Z., Pourtois, G. & Brédas, J. L. Spin-orbit coupling and intersystem crossing in conjugated polymers: a configuration interaction description. J. Phys. Chem. A 105, 3899–3907 (2001)
Takase, M., Enkelmann, V., Sebastiani, D., Baumgarten, M. & Müllen, K. Annularly fused hexapyrrolohexaazacoronenes: an extended π system with multiple interior nitrogen atoms displays stable oxidation states. Angew. Chem. Int. Ed. 46, 5524–5527 (2007)
Uno, H. et al. Preparation of highly conjugated oligoaza-PAHs based on the oxidative intramolecular coupling of bicyclo[2.2.2]octadiene-fused pyrrole. Heterocycles 82, 791–802 (2010)
Acknowledgements
This work was supported by the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST) and the International Institute for Carbon Neutral Energy Research (WPI-I2CNER), sponsored by the Japanese Ministry of Education, Culture, Sports, Science and Technology. H.U. acknowledges a Grand-in-Aid for JSPS Fellows. We thank H. Nakanotani, J.-i. Nishide, and H. Miyazaki for their assistance with this research. We also thank K. Tokumaru, H. Sasabe, W. Potscavage and M. Gábor for their assistance with preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
H.U. performed the molecular design. H.U. and H.N. performed the synthetic work. K.S. performed the computational experiments. H.U. and K.G. measured the photoluminescence and electroluminescence characteristics of the compounds. All authors wrote the paper. C.A. thought of the TADF concept and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-2 and Supplementary Tables 1-4. (PDF 266 kb)
Rights and permissions
About this article
Cite this article
Uoyama, H., Goushi, K., Shizu, K. et al. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012). https://doi.org/10.1038/nature11687
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11687
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.