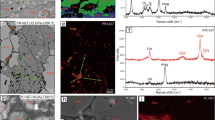
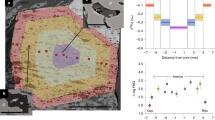
Abstract
Determining the oxygen fugacity of Earth’s silicate mantle is of prime importance because it affects the speciation and mobility of volatile elements in the interior and has controlled the character of degassing species from the Earth since the planet’s formation1. Oxygen fugacities recorded by garnet-bearing peridotite xenoliths from Archaean lithosphere are of particular interest, because they provide constraints on the nature of volatile-bearing metasomatic fluids and melts active in the oldest mantle samples, including those in which diamonds are found2,3. Here we report the results of experiments to test garnet oxythermobarometry equilibria4,5 under high-pressure conditions relevant to the deepest mantle xenoliths. We present a formulation for the most successful equilibrium and use it to determine an accurate picture of the oxygen fugacity through cratonic lithosphere. The oxygen fugacity of the deepest rocks is found to be at least one order of magnitude more oxidized than previously estimated. At depths where diamonds can form, the oxygen fugacity is not compatible with the stability of either carbonate- or methane-rich liquid but is instead compatible with a metasomatic liquid poor in carbonate and dominated by either water or silicate melt. The equilibrium also indicates that the relative oxygen fugacity of garnet-bearing rocks will increase with decreasing depth during adiabatic decompression. This implies that carbon in the asthenospheric mantle will be hosted as graphite or diamond but will be oxidized to produce carbonate melt through the reduction of Fe3+ in silicate minerals during upwelling. The depth of carbonate melt formation will depend on the ratio of Fe3+ to total iron in the bulk rock. This ‘redox melting’ relationship has important implications for the onset of geophysically detectable incipient melting and for the extraction of carbon dioxide from the mantle through decompressive melting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kasting, J. F., Eggler, D. H. & Raeburn, S. P. Mantle redox evolution and the oxidation state of the Archean atmosphere. J. Geol. 101, 245–257 (1993)
Luth, R. W., Virgo, D., Boyd, F. R. & Wood, B. J. Ferric iron in mantle-derived garnets. Contrib. Mineral. Petrol. 104, 56–72 (1990)
Woodland, A. B. & Koch, M. Variation in oxygen fugacity with depth in the upper mantle beneath Kaapvaal craton, South Africa. Earth Planet. Sci. Lett. 214, 295–310 (2003)
O’Neill, H. St C. & Wall, V. J. The olivine-orthopyroxene-spinel oxygen geobarometer, the nickel precipitation curve, and the oxygen fugacity of the Earth’s upper mantle. J. Petrol. 28, 1169–1191 (1987)
Wood, B. J. Oxygen barometry of spinel peridotites. Rev. Mineral. Geochem. 25, 417–432 (1991)
Ballhaus, C. & Frost, B. R. The generation of oxidized CO2-bearing basaltic melts from reduced CH4-bearing upper mantle sources. Geochem. Cosmochem. Acta 58, 4931–4940 (1994)
Gudmundsson, G. & Wood, B. J. Experimental tests of garnet peridotite oxygen barometry. Contrib. Mineral. Petrol. 119, 56–67 (1995)
Frost, D. J. & McCammon, C. A. The redox state of the Earth’s mantle. Annu. Rev. Earth Planet. Sci. 36, 389–420 (2008)
Ballhaus, C. Is the upper mantle metal-saturated? Earth Planet. Sci. Lett. 132, 75–86 (1995)
O’Neill St C, H., Rubie, D. C., Canil, D., Geiger, C. A. & Ross, C. R. in Evolution of the Earth and Planets (eds Takahashi, E., Jeanloz, R. & Rubie, D. C.) 74–88 (Geophys. Monogr. 74, American Geophysical Union, 1993)
Woodland, A. B. & O’Neill, H. St C. Thermodynamic data for Fe-bearing phases obtained using noble metal alloys as redox sensors. Geochim. Cosmochim. Acta 61, 4359–4366 (1997)
Woodland, A. B. & O’Neill, H. St C. Synthesis and stability of Fe3Fe2 3+Si3O12 garnet and phase relations with Fe3Al2Si3O12-Fe3Fe2 3+Si3O12 solutions. Am. Mineral. 78, 1000–1013 (1993)
Holland, T. & Powell, R. An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. J. Metamorph. Geol. 29, 333–383 (2011)
Canil, D. & O’Neill, H. St C. Distribution of ferric iron in some upper-mantle assemblages. J. Petrol. 37, 609–635 (1996)
McCammon, C. A. & Kopylova, M. G. A redox profile of the Slave mantle and oxygen fugacity control in the cratonic mantle. Contrib. Mineral. Petrol. 148, 55–68 (2004)
Lazarov, M., Woodland, A. B. & Brey, G. P. Thermal state and redox conditions of the Kaapvaal mantle: a study of xenoliths from the Finsch mine, South Africa. Lithos 112S, 913–923 (2009)
Creighton, S. et al. Oxidation of the Kaapvaal lithospheric mantle driven by metasomatism. Contrib. Mineral. Petrol. 157, 491–504 (2009)
Creighton, S., Stachel, T., Eichenberg, D. & Luth, R. W. Oxidation state of the lithospheric mantle beneath Diavik diamond mine, central Slave craton, NWT, Canada. Contrib. Mineral. Petrol. 159, 645–657 (2010)
Yaxley, G. M., Berry, A. J., Kamenetsky, V. S., Woodland, A. B. & Golovin, A. V. An oxygen fugacity profile through the Siberian Craton-Fe K-edge XANES determinations of Fe3+/ΣFe in garnets in peridotite xenoliths from the Udachnaya East kimberlite. Lithos 140–141, 142–151 (2012)
Eggler, D. H. & Baker, D. R. in High-Pressure Research in Geophysics (eds Akimoto, S. & Manghnani, M. H.) 237–250 (Springer, 1982)
Dasgupta, R. & Hirschmann, M. M. The deep carbon cycle and melting in Earth’s interior. Earth Planet. Sci. Lett. 298, 1–13 (2010)
Stagno, V. & Frost, D. J. Carbon speciation in the asthenosphere: experimental measurements of the redox conditions at which carbonate-bearing melts coexist with graphite or diamond in peridotite assemblages. Earth Planet. Sci. Lett. 300, 72–84 (2010)
Peslier, A. H., Woodland, A. B., Bell, D. R. & Lazarov, M. Olivine water contents in the continental lithosphere and the longevity of cratons. Nature 467, 78–81 (2010)
McDonough, W. F. & Sun, S.-s. The composition of the Earth. Chem. Geol. 120, 223–253 (1995)
Bézos, A. & Humler, E. The Fe3+/ΣFe ratios of MORB glasses and their implications for mantle melting. Geochim. Cosmochim. Acta 69, 711–725 (2005)
Blundy, J. D., Brodholt, J. P. & Wood, B. J. Carbon-fluid equilibria and the oxidation state of the upper mantle. Nature 349, 321–324 (1991)
Saal, A. E., Hauri, E., Langmuir, C. H. & Perfit, M. R. Vapour undersaturation in primitive mid-ocean-ridge basalts and the volatile content of Earth’s upper mantle. Nature 419, 451–455 (2002)
Gaillard, F., Malki, M., Iacono-Marziano, G., Pichavant, M. & Scaillet, B. Carbonatite melts and electrical conductivity in the asthenosphere. Science 322, 1363–1365 (2008)
Gu, Y. J., Lerner-Lam, A. L., Dziewonski, A. M. & Ekstrom, G. Deep structure and seismic anisotropy beneath the East Pacific Rise. Earth Planet. Sci. Lett. 232, 259–272 (2005)
Rohrbach, A. et al. Metal saturation in the upper mantle. Nature 449, 456–458 (2007)
Rohrbach, A. & Schmidt, M. W. Redox freezing and melting in the Earth’s deep mantle resulting from carbon-iron redox coupling. Nature 472, 209–212 (2011)
McKenzie, D. The extraction of magma from the crust and mantle. Earth Planet. Sci. Lett. 74, 81–91 (1985)
Campbell, I. H. Large igneous provinces and the plume hypothesis. Elements 1, 265–269 (2005)
O’Neill, H. St C. & Wood, B. J. An experimental study of Fe-Mg partitioning between garnet and olivine and its calibration as a geothermometer. Contrib. Mineral. Petrol. 70, 59–70 (1979)
Balta, J. B., Asimow, P. D. & Mosenfelder, J. L. Hydrous, low-carbon melting of garnet peridotite. J. Petrol. 52, 2079–2105 (2011)
McCammon, C. A. A Mössbauer milliprobe: practical considerations. Hyperfine Interact. 92, 1235–1239 (1994)
Prescher, C., McCammon, C. & Dubrovinsky, L. MossA: a program for analyzing energy-domain Mossbauer spectra from conventional and synchrotron sources. J. Appl. Crystallogr. 45, 329–331 (2012)
Amthauer, G., Annersten, H. & Hafner, S. S. The Mössbauer spectrum of 57Fe in silicate garnets. Z. Kristallogr. 143, 14–55 (1976)
Schwerdtfeger, K. & Zwell, L. Activities in solid iridium-iron and rhodium-iron alloys at 1200°C. Trans. Metall. Soc. AIME 242, 631–633 (1968)
Swartzendruber, L. J. The Fe-Ir (iron-iridium) system. Bull. Alloy Phase Diagr. 5, 48–52 (1984)
Wiser, N. & Wood, B. J. Experimental determination of activities in Fe-Mg olivine at 1400K. Contrib. Mineral. Petrol. 108, 146–153 (1991)
Ganguly, J., Cheng, C. & Tirone, M. Thermodynamics of aluminosilicate garnet solid solutions: new experimental data, an optimized model, and thermometric applications. Contrib. Mineral. Petrol. 126, 137–151 (1996)
Wood, B. J. & Nicholls, J. The thermodynamical properties of reciprocal solid solutions. Contrib. Mineral. Petrol. 66, 389–400 (1978)
Acknowledgements
Financial support was provided to V.S. by the European Commission under the Marie Curie Action for Early Stage Training of Researchers within the 6th Framework Programme (contract number MEST-CT-2005-019700) and by the German Science Foundation (grant FR1555/5-1).
Author information
Authors and Affiliations
Contributions
V.S. and D.J.F. wrote the paper. V.S. performed most of the experiments, analytical measurements and calculations. D.O.O. performed high-pressure experiments. C.A.M. collected and interpreted Mössbauer data. D.J.F. developed the thermodynamic model.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data and Supplementary Figures 1-2. (PDF 439 kb)
Supplementary Tables
This file contains Supplementary Tables 1-5. (XLS 38 kb)
Supplementary Data
This file contains the thermodynamic model developed in this study and also the calculation for the fo2 of a garnet peridotite assemblage. (XLS 95 kb)
Rights and permissions
About this article
Cite this article
Stagno, V., Ojwang, D., McCammon, C. et al. The oxidation state of the mantle and the extraction of carbon from Earth’s interior. Nature 493, 84–88 (2013). https://doi.org/10.1038/nature11679
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11679
This article is cited by
-
The Fe(Ni)–C–N-phase diagram at 10 GPa—implications for nitrogen and carbon storage in the deep mantle
Contributions to Mineralogy and Petrology (2024)
-
Genesis and evolution of kimberlites
Nature Reviews Earth & Environment (2023)
-
Imperfections in natural diamond: the key to understanding diamond genesis and the mantle
La Rivista del Nuovo Cimento (2023)
-
Redox state of the Dharwar craton root as inferred from eclogite and peridotite sourced mantle cargo, with implications for kimberlite and lamproite magma formation
Contributions to Mineralogy and Petrology (2023)
-
An experimental investigation of factors controlling the oxygen content of sulphide melts in the Earth’s upper mantle
Contributions to Mineralogy and Petrology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.