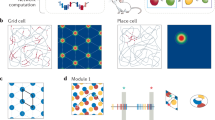
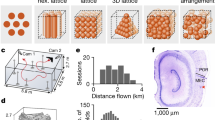
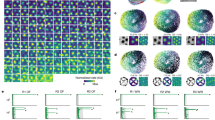
Abstract
The medial entorhinal cortex (MEC) is part of the brain’s circuit for dynamic representation of self-location. The metric of this representation is provided by grid cells, cells with spatial firing fields that tile environments in a periodic hexagonal pattern. Limited anatomical sampling has obscured whether the grid system operates as a unified system or a conglomerate of independent modules. Here we show with recordings from up to 186 grid cells in individual rats that grid cells cluster into a small number of layer-spanning anatomically overlapping modules with distinct scale, orientation, asymmetry and theta-frequency modulation. These modules can respond independently to changes in the geometry of the environment. The discrete topography of the grid-map, and the apparent autonomy of the modules, differ from the graded topography of maps for continuous variables in several sensory systems, raising the possibility that the modularity of the grid map is a product of local self-organizing network dynamics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mountcastle, V. B. Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J. Neurophysiol. 20, 408–434 (1957)
Hubel, D. H. & Wiesel, T. Receptive fields, binocular interaction, and functional architecture of cat striate cortex. J. Physiol. (Lond.) 160, 106–154 (1962)
Hubel, D. H. & Wiesel, T. Functional architecture of macaque monkey visual cortex. Proc. R. Soc. Lond. B Biol. Sci. 198, 1–59 (1977)
Hubel, D. H. & Wiesel, T. Sequence regularity and geometry of orientation columns in the monkey striate cortex. J. Comp. Neurol. 158, 267–293 (1974)
Blasdel, G. G. & Salama, G. Voltage sensitive dyes reveal a modular organization in monkey striate cortex. Nature 321, 579–585 (1986)
Bonhoeffer, T. & Grinvald, A. Orientation columns in cat are organized in pin-wheel like patterns. Nature 353, 429–431 (1991)
Ohki, K. et al. Highly ordered arrangement of single neurons in orientation pinwheels. Nature 442, 925–928 (2006)
Payne, B. R., Berman, N. & Murphy, E. H. Organization of direction preferences in cat visual cortex. Brain Res. 211, 445–450 (1981)
Tolhurst, D. J., Dean, A. F. & Thompson, I. D. Preferred direction of movement as an element in the organization of cat visual cortex. Exp. Brain Res. 44, 340–342 (1981)
Weliky, M., Bosking, W. H. & Fitzpatrick, D. A systematic map of direction preference in primary visual cortex. Nature 379, 725–728 (1996)
Ohki, K., Chung, S., Ch’ng, Y. H., Kara, P. & Reid, R. C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005)
Sperry, R. W. Visuomotor coordination in the newt (Triturus viridescens) after regeneration of the optic nerve. J. Comp. Neurol. 79, 33–55 (1943)
Drescher, U. et al. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell 82, 359–370 (1995)
Cheng, H. J., Nakamoto, M., Bergemann, A. D. & Flanagan, J. G. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell 82, 371–381 (1995)
O’Keefe, J. & Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 (1971)
O’Keefe, J. & Nadel, L. The Hippocampus as a Cognitive Map (Oxford Univ. Press, 1978)
Moser, E. I., Kropff, E. & Moser, M.-B. Place cells, grid cells, and the brain’s spatial representation system. Annu. Rev. Neurosci. 31, 69–89 (2008)
Fyhn, M., Molden, S., Witter, M. P., Moser, E. I. & Moser, M. B. Spatial representation in the entorhinal cortex. Science 305, 1258–1264 (2004)
Hafting, T., Fyhn, M., Molden, S., Moser, M.-B. & Moser, E. I. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806 (2005)
Sargolini, F. et al. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312, 758–762 (2006)
McNaughton, B. L., Battaglia, F. P., Jensen, O., Moser, E. I. & Moser, M.-B. Path integration and the neural basis of the ‘cognitive map’. Nature Rev. Neurosci. 7, 663–678 (2006)
Giocomo, L. M., Moser, M.-B. & Moser, E. W. I. Computational models of grid cells. Neuron 71, 589–603 (2011)
Fuhs, M. C. & Touretzky, D. S. A spin glass model of path integration in rat medial entorhinal cortex. J. Neurosci. 26, 4266–4276 (2006)
Burgess, N., Barry, C. & O’Keefe, J. An oscillatory interference model of grid cell firing. Hippocampus 17, 801–812 (2007)
Kropff, E. & Treves, A. The emergence of grid cells: intelligent design or just adaptation? Hippocampus 18, 1256–1269 (2008)
Burak, Y. & Fiete, I. R. Accurate path integration in continuous attractor network models of grid cells. PLOS Comput. Biol. 5, e1000291 (2009)
Zilli, E. A. & Hasselmo, M. E. Coupled noisy spiking neurons as velocity-controlled oscillators in a model of grid cell spatial firing. J. Neurosci. 30, 13850–13860 (2010)
Mhatre, H., Gorchetchnikov, A. & Grossberg, S. Grid cell hexagonal patterns formed by fast self-organized learning within entorhinal cortex. Hippocampus 22, 320–334 (2012)
Witter, M. P. & Moser, E. I. Spatial representation and the architecture of the entorhinal cortex. Trends Neurosci. 29, 671–678 (2006)
Barry, C., Hayman, R., Burgess, N. & Jeffery, K. J. Experience-dependent rescaling of entorhinal grids. Nature Neurosci. 10, 682–684 (2007)
Solstad, T., Boccara, C. N., Kropff, E., Moser, M.-B. & Moser, E. I. Representation of geometric borders in the entorhinal cortex. Science 322, 1865–1868 (2008)
Brun, V. H. et al. Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex. Hippocampus 18, 1200–1212 (2008)
Hafting, T., Fyhn, M., Bonnevie, T., Moser, M.-B. & Moser, E. I. Hippocampus-independent phase precession in entorhinal grid cells. Nature 453, 1248–1252 (2008)
Jeewajee, A., Barry, C., O’Keefe, J. & Burgess, N. Grid cells and theta as oscillatory interference: electrophysiological data from freely moving rats. Hippocampus 18, 1175–1185 (2008)
Fyhn, M., Hafting, T., Treves, A., Moser, M. B. & Moser, E. I. Hippocampal remapping and grid realignment in entorhinal cortex. Nature 446, 190–194 (2007)
Yartsev, M. M., Witter, M. P. & Ulanovsky, N. Grid cells without theta oscillations in the entorhinal cortex of bats. Nature 479, 103–107 (2011)
Krupic, J., Burgess, N. & O’Keefe, J. Neural representations of location composed of spatially periodic bands. Science 337, 853–857 (2012)
Doeller, C. F., Barry, C. & Burgess, N. Evidence for grid cells in a human memory network. Nature 463, 657–661 (2010)
Hevner, R. F. & Wong-Riley, M. T. Entorhinal cortex of the human, monkey, and rat: metabolic map as revealed by cytochrome oxidase. J. Comp. Neurol. 326, 451–469 (1992)
Burgalossi, A. et al. Microcircuits of functionally identified neurons in the rat medial entorhinal cortex. Neuron 70, 773–786 (2011)
Ikeda, J., Mori, K., Oka, S. & Watanbe, Y. A columnar arrangement of dendritic processes of entorhinal cortex neurons revealed by a monoclonal antibody. Brain Res. 505, 176–179 (1989)
Couey, J. J. et al. Medical entorhinal cortex layer ii stellate cells are embedded within a recurrent inhibitory network. Soc. Necuosci. abstr. 702.06 (2012)
Langston, R. F. et al. Development of the spatial representation system in the rat. Science 328, 1576–1580 (2010)
Wills, T. J., Cacucci, F., Burgess, N. & O’Keefe, J. Development of the hippocampal cognitive map in preweanling rats. Science 328, 1573–1576 (2010)
Hasselmo, M. E., Giocomo, L. M. & Zilli, E. A. Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons. Hippocampus 17, 1252–1271 (2007)
Blair, H. T., Welday, A. C. & Zhang, K. Scale-invariant memory representations emerge from moiré interference between grid fields that produce theta oscillations: a computational model. J. Neurosci. 27, 3211–3229 (2007)
Sreenivasan, S. & Fiete, I. Grid cells generate an analog error-correcting code for singularly precise neural computation. Nature Neurosci. 14, 1330–1337 (2011)
Monaco, J. D. & Abbott, L. F. Modular realignment of entorhinal grid cell activity as a basis for hippocampal remapping. J. Neurosci. 31, 9414–9425 (2011)
Muller, R. U. & Kubie, J. L. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J. Neurosci. 7, 1951–1968 (1987)
Colgin, L. L., Moser, E. I. & Moser, M.-B. Understanding memory through hippocampal remapping. Trends Neurosci. 31, 469–477 (2008)
Acknowledgements
We thank A. M. Amundgård, K. Haugen, K. Jenssen, E. Kråkvik, R. Skjerpeng, and H. Waade for technical assistance, J. Whitlock for implanting an animal in the tangential group, and T. Bonhoeffer, B. Dunn, B. McNaughton, Y. Roudi, A. Treves and A. Witoelar for helpful discussion. The work was supported by an Advanced Investigator Grant from the European Research Council (‘CIRCUIT’, Grant Agreement no. 232608), the Kavli Foundation and the Centre of Excellence scheme of the Research Council of Norway.
Author information
Authors and Affiliations
Contributions
T.St., H.S., T.So., M.-B.M. and E.I.M. designed experiments and analyses; H.S., T.St. and T.So. implanted tetrodes; H.S. recorded multisite data; T.So. and K.F. tested animals with tangential implants; T.St. performed the majority of the analyses; and T.St. and E.I.M. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Materials and Methods and Supplementary References. (PDF 388 kb)
Supplementary Figures
This file contains Supplementary Figures 1-20. (PDF 10537 kb)
Rights and permissions
About this article
Cite this article
Stensola, H., Stensola, T., Solstad, T. et al. The entorhinal grid map is discretized. Nature 492, 72–78 (2012). https://doi.org/10.1038/nature11649
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11649
This article is cited by
-
Subicular neurons encode concave and convex geometries
Nature (2024)
-
A consistent map in the medial entorhinal cortex supports spatial memory
Nature Communications (2024)
-
Phase information is conserved in sparse, synchronous population-rate-codes via phase-to-rate recoding
Nature Communications (2023)
-
Coordinated drift of receptive fields in Hebbian/anti-Hebbian network models during noisy representation learning
Nature Neuroscience (2023)
-
A unifying perspective on neural manifolds and circuits for cognition
Nature Reviews Neuroscience (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.