Abstract
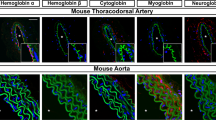
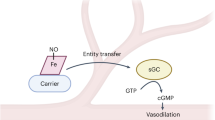
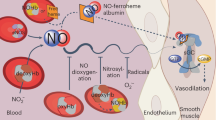
Models of unregulated nitric oxide (NO) diffusion do not consistently account for the biochemistry of NO synthase (NOS)-dependent signalling in many cell systems1,2,3. For example, endothelial NOS controls blood pressure, blood flow and oxygen delivery through its effect on vascular smooth muscle tone4, but the regulation of these processes is not adequately explained by simple NO diffusion from endothelium to smooth muscle3,5. Here we report a new model for the regulation of NO signalling by demonstrating that haemoglobin (Hb) α (encoded by the HBA1 and HBA2 genes in humans) is expressed in human and mouse arterial endothelial cells and enriched at the myoendothelial junction, where it regulates the effects of NO on vascular reactivity. Notably, this function is unique to Hb α and is abrogated by its genetic depletion. Mechanistically, endothelial Hb α haem iron in the Fe3+ state permits NO signalling, and this signalling is shut off when Hb α is reduced to the Fe2+ state by endothelial cytochrome b5 reductase 3 (CYB5R3, also known as diaphorase 1)6. Genetic and pharmacological inhibition of CYB5R3 increases NO bioactivity in small arteries. These data reveal a new mechanism by which the regulation of the intracellular Hb α oxidation state controls NOS signalling in non-erythroid cells. This model may be relevant to haem-containing globins in a broad range of NOS-containing somatic cells7,8,9,10,11,12,13.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lim, K. H., Ancrile, B. B., Kashatus, D. F. & Counter, C. M. Tumour maintenance is mediated by eNOS. Nature 452, 646–649 (2008)
Hess, D. T., Matsumoto, A., Kim, S. O., Marshall, H. E. & Stamler, J. S. Protein S-nitrosylation: purview and parameters. Nature Rev. Mol. Cell Biol. 6, 150–166 (2005)
Bolotina, V. M., Najibi, S., Palacino, J. J., Pagano, P. J. & Cohen, R. A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 368, 850–853 (1994)
Shesely, E. G. et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA 93, 13176–13181 (1996)
Straub, A. C. et al. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler. Thromb. Vasc. Biol. 31, 399–407 (2011)
Hultquist, D. E. & Passon, P. G. Catalysis of methaemoglobin reduction by erythrocyte cytochrome B5 and cytochrome B5 reductase. Nat. New Biol. 229, 252–254 (1971)
Newton, D. A., Rao, K. M., Dluhy, R. A. & Baatz, J. E. Hemoglobin is expressed by alveolar epithelial cells. J. Biol. Chem. 281, 5668–5676 (2006)
Nishi, H. et al. Hemoglobin is expressed by mesangial cells and reduces oxidant stress. J. Am. Soc. Nephrol. 19, 1500–1508 (2008)
Liu, L., Zeng, M. & Stamler, J. S. Hemoglobin induction in mouse macrophages. Proc. Natl Acad. Sci. USA 96, 6643–6647 (1999)
Schelshorn, D. W. et al. Expression of hemoglobin in rodent neurons. J. Cereb. Blood Flow Metab. 29, 585–595 (2009)
Halligan, K. E., Jourd’heuil, F. L. & Jourd’heuil, D. Cytoglobin is expressed in the vasculature and regulates cell respiration and proliferation via nitric oxide dioxygenation. J. Biol. Chem. 284, 8539–8547 (2009)
Brunori, M. et al. Neuroglobin, nitric oxide, and oxygen: functional pathways and conformational changes. Proc. Natl Acad. Sci. USA 102, 8483–8488 (2005)
Flogel, U., Merx, M. W., Godecke, A., Decking, U. K. & Schrader, J. Myoglobin: a scavenger of bioactive NO. Proc. Natl Acad. Sci. USA 98, 735–740 (2001)
Dora, K. A., Doyle, M. P. & Duling, B. R. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc. Natl Acad. Sci. USA 94, 6529–6534 (1997)
Angelo, M., Hausladen, A., Singel, D. J. & Stamler, J. S. Interactions of NO with hemoglobin: from microbes to man. Methods Enzymol. 436, 131–168 (2008)
Gladwin, M. T., Lancaster, J. R., Jr, Freeman, B. A. & Schechter, A. N. Nitric oxide’s reactions with hemoglobin: a view through the SNO-storm. Nature Med. 9, 496–500 (2003)
Palmer, R. M., Ferrige, A. G. & Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524–526 (1987)
Ignarro, L. J., Adams, J. B., Horwitz, P. M. & Wood, K. S. Activation of soluble guanylate cyclase by NO-hemoproteins involves NO-heme exchange. Comparison of heme-containing and heme-deficient enzyme forms. J. Biol. Chem. 261, 4997–5002 (1986)
Ignarro, L. J., Byrns, R. E., Buga, G. M. & Wood, K. S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ. Res. 61, 866–879 (1987)
Cassoly, R. & Gibson, Q. Conformation, co-operativity and ligand binding in human hemoglobin. J. Mol. Biol. 91, 301–313 (1975)
Doyle, M. P. & Hoekstra, J. W. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J. Inorg. Biochem. 14, 351–358 (1981)
Eich, R. F. et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry 35, 6976–6983 (1996)
Sharma, V. S., Traylor, T. G., Gardiner, R. & Mizukami, H. Reaction of nitric oxide with heme proteins and model compounds of hemoglobin. Biochemistry 26, 3837–3843 (1987)
Tejero, J. et al. Low NO concentration-dependence of the reductive nitrosylation reaction of hemoglobin. J. Biol. Chem. 287, 18262–18274 (2012)
Angelo, M., Singel, D. J. & Stamler, J. S. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc. Natl Acad. Sci. USA 103, 8366–8371 (2006)
Lee, E. & Kariya, K. Propylthiouracil, a selective inhibitor of NADH-cytochrome b5 reductase. FEBS Lett. 209, 49–51 (1986)
Lam, Y. H. & Tang, M. H. Middle cerebral artery Doppler study in fetuses with homozygous α-thalassaemia-1 at 12–13 weeks of gestation. Prenat. Diagn. 22, 56–58 (2002)
Fregly, M. J. & Hood, C. I. Physiologic and anatomic effects of prophylthiouracil on normal and hypertensive rats. Circ. Res. 7, 486–496 (1959)
Heberlein, K. R. et al. Plasminogen activator inhibitor-1 regulates myoendothelial junction formation. Circ. Res. 106, 1092–1102 (2010)
Davalos, A. et al. Quantitative proteomics of caveolin-1-regulated proteins: characterization of polymerase I and transcript release factor/CAVIN-1 in endothelial cells. Mol Cell Proteomics 9, 2109–2124 (2010)
Acknowledgements
We thank the Advanced Microscopy and Histology core at the University of Virginia and the Yale Proteomic Facility. We acknowledge V. Balasubramaniam, S. Lewis and D. Singel for discussions of the data and B. Duling for critical evaluation of experiments and the manuscript. We also thank M. Weiss for the Hb α-stabilizing protein antibody and for discussions. This work was supported by an American Heart Association Scientist Development Grant (B.E.I.), National Institutes of Health grants HL088554 (B.E.I.), HL107963 (B.E.I.) HL059337 (B.G.), HL101871 (B.G.), HL112904 (A.C.S.) and HL007284 (A.W.L. and A.C.S.). M.B. and S.R.J. were supported by American Heart Association postdoctoral fellowships, and A.W.L. and M.Y.L. were supported by American Heart Association predoctoral fellowships.
Author information
Authors and Affiliations
Contributions
A.C.S. performed most of the experiments and data analysis. A.W.L. performed vessel transfections. Vascular reactivity was executed by A.W.L. and M.B. S.R.J. carried out immunofluorescence studies and S.T.D. assisted in NO diffusion and consumption assays. M.Y.L. and P.S.B. performed real-time PCR experiments, and A.K.B. helped with all cell culture experiments. L.C. performed the modelling experiments. B.G. helped with experimental design, provided use of the NO analyser and NO, and assisted with final manuscript preparation. B.E.I. initiated, directed and supported the work through all levels of development. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-18, Supplementary Tables 1-2 and Supplementary Methods, which include Supplementary References and Tables 1-3. (PDF 4557 kb)
Rights and permissions
About this article
Cite this article
Straub, A., Lohman, A., Billaud, M. et al. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature 491, 473–477 (2012). https://doi.org/10.1038/nature11626
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11626
This article is cited by
-
CYB5R3 functions as a tumor suppressor by inducing ER stress-mediated apoptosis in lung cancer cells via the PERK-ATF4 and IRE1α-JNK pathways
Experimental & Molecular Medicine (2024)
-
Thiol-catalyzed formation of NO-ferroheme regulates intravascular NO signaling
Nature Chemical Biology (2023)
-
Signaling pathways in vascular function and hypertension: molecular mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
NO-ferroheme is a signaling entity in the vasculature
Nature Chemical Biology (2023)
-
Maternal high-fat diet in mice induces cerebrovascular, microglial and long-term behavioural alterations in offspring
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.