Abstract
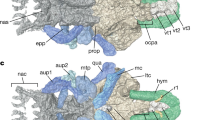
Teeth and jaws constitute a model of the evolutionary developmental biology concept of modularity1 and they have been considered the key innovations underpinning a classic example of adaptive radiation2. However, their evolutionary origins are much debated. Placoderms comprise an extinct sister clade3 or grade4,5 to the clade containing chondrichthyans and osteichthyans, and although they clearly possess jaws, previous studies have suggested that they lack teeth6,7,8, that they possess convergently evolved tooth-like structures9,10,11 or that they possess true teeth12. Here we use synchrotron radiation X-ray tomographic microscopy (SRXTM)13 of a developmental series of Compagopiscis croucheri (Arthrodira) to show that placoderm jaws are composed of distinct cartilages and gnathal ossifications in both jaws, and a dermal element in the lower jaw. The gnathal ossification is a composite of distinct teeth that developed in succession, polarized along three distinct vectors, comparable to tooth families. The teeth are composed of dentine and bone, and show a distinct pulp cavity that is infilled centripetally as development proceeds. This pattern is repeated in other placoderms, but differs from the structure and development of tooth-like structures in the postbranchial lamina and dermal skeleton of Compagopiscis and other placoderms. We interpret this evidence to indicate that Compagopiscis and other arthrodires possessed teeth, but that tooth and jaw development was not developmentally or structurally integrated in placoderms. Teeth did not evolve convergently among the extant and extinct classes of early jawed vertebrates but, rather, successional teeth evolved within the gnathostome stem-lineage soon after the origin of jaws. The chimaeric developmental origin of this model of modularity reflects the distinct evolutionary origins of teeth and of component elements of the jaws.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Atchley, W. R. & Hall, B. K. A model for development and evolution of complex morphological structures and its application to the mammalian mandible. Biol. Rev. Camb. Philos. Soc. 66, 101–157 (1991)
Gans, C. & Northcutt, R. G. Neural crest and the origin of the vertebrates: a new head. Science 220, 268–273 (1983)
Young, G. C. Placoderms (armored fish): dominant vertebrates of the Devonian Period. Annu. Rev. Earth Planet. Sci. 38, 523–550 (2010)
Brazeau, M. D. The braincase and jaws of a Devonian ‘acanthodian’ and modern gnathostome origins. Nature 457, 305–308 (2009)
Davis, S. P. et al. Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes. Nature 486, 247–250 (2012)
Reif, W.-E. Evolution of dermal skeleton and dentition in vertebrates: The odontode regulation theory. Evol. Biol. 15, 287–368 (1982)
Burrow, C. J. Comment on “Separate evolutionary origins of teeth from evidence in fossil jawed vertebrates”. Science 300, 1661 (2003)
Young, G. C. Did placoderm fish have teeth? J. Vertebr. Paleontol. 23, 987–990 (2003)
Johanson, Z. & Smith, M. M. Placoderm fishes, pharyngeal denticles, and the vertebrate dentition. J. Morphol. 257, 289–307 (2003)
Johanson, Z. & Smith, M. M. Origin and evolution of gnathostome dentitions: a question of teeth and pharyngeal denticles in placoderms. Biol. Rev. Camb. Philos. Soc. 80, 303–345 (2005)
Smith, M. M. & Johanson, Z. Separate evolutionary origin of teeth from evidence in fossil jawed vertebrates. Science 299, 1235–1236 (2003)
Ørvig, T. Histologic studies of ostracoderms, placoderms and fossil elasmobranchs 3. Structure and growth of gnathalia of certain arthrodires. Zool. Scr. 9, 141–159 (1980)
Donoghue, P. C. J. et al. Synchrotron X-ray tomographic microscopy of fossil embryos. Nature 442, 680–683 (2006)
Lelièvre, H. Description of Maideria falipoui n.g., n.sp., a long snouted brachythoracid (Vertebrata, Placodermi, Arthrodira) from the Givetian of Maider (South Morocco), with a phylogenetic analysis of primitive brachythoracids. Bulletin du Muséum National d'Histoire Naturelle, Paris 17, 163–207 (1995)
Young, G. C. The relationships of placoderm fishes. Zool. J. Linn. Soc. 88, 1–57 (1986)
Downs, J. P. & Donoghue, P. C. J. Skeletal histology of Bothriolepis canadensis (Placodermi, Antiarchi) and evolution of the skeleton at the origin of jawed vertebrates. J. Morphol. 270, 1364–1380 (2009)
Huysseune, A. et al. Evolutionary and developmental origins of the vertebrate dentition. J. Anat. 214 465–476 (2009) Medline
Huysseune, A. et al. Unique and shared gene expression patterns in Atlantic salmon (Salmo salar) tooth development. Dev. Genes Evol. 218, 427–437 (2008)
Fraser, G. J. et al. Developmental and evolutionary origins of the vertebrate dentition: Molecular controls for spatio-temporal organisation of tooth sites in osteichthyans. J. Exp. Zool. B Mol. Dev. Evol. 306, 183–203 (2006)
Ørvig, T. Acanthodian dentition and its bearing on the relationships of the group. Palaeontographica (Abt. A) 143, 119–150 (1973)
Finarelli, J. A. & Coates, M. I. First tooth-set outside the jaws in a vertebrate. Proc. R. Soc. B 279, 775–779 (2012)
Smith, M. M. The pattern of histogenesis and growth of tooth plates in larval stages of extant lungfish. J. Anat. 140, 627–643 (1985)
Fraser, G. J. et al. The odontode explosion: the origin of tooth-like structures in vertebrates. Bioessays 32, 808–817 (2010)
Rücklin, M. et al. Teeth before jaws? Comparative analysis of the structure and development of the external and internal scales in the extinct jawless vertebrate Loganellia scotica. Evol. Dev. 13, 523–532 (2011)
Soukup, V. et al. Dual epithelial origin of vertebrate oral teeth. Nature 455, 795–798 (2008)
Stampanoni, M. et al. TOMCAT: A beamline for TOmographic Microscopy and Coherent rAdiology experimenTs. Synchrotron Radiation Instrumentation, Pts 1 and 2 879 848–851 http://www.osti.gov/energycitations/product.biblio.jsp?osti_id=21052651 (2007)
Goujet, D. & Young, G. C. Interrelationships of placoderms revisited. Geobios 19, 89–95 (1995)
Goujet, D. & Young, G. C. in Recent Advances in the Origin and Early Radiation of Vertebrates (eds Arratia, G., Wilson, M. V. H. & Cloutier, R. ) 109–126 (Pfeil, 2004)
Marone, F. & Stampanoni, M. Regridding reconstruction algorithm for real time tomographic imaging. J. Synchrotron Radiat. 19, 1–9 (2012)
Acknowledgements
We thank S. Bengtson, J. Cunningham, D. Murdock, S. Giles and A. Hetherington for help at the TOMCAT beamline; K. Robson-Brown for help at the Micro-CT, and H. Lélièvre and G. Clément for loan of specimens. The study was funded by EU grant FP7 MC-IEF (to M.R. and P.C.J.D.), Australian Research Council Grant DP 110101127 (to Z.J. and K.T.), Natural Environment Research Council grant NE/G016623/1 (to P.C.J.D.) and the Paul Scherrer Institut (P.C.J.D.).
Author information
Authors and Affiliations
Contributions
M.R., P.C.J.D. and Z.J. conceived the project. K.T. acid-prepared WAM specimens. M.R., P.C.J.D., F.M. and M.S. collected the data. M.R. analysed the data. All authors contributed to the interpretation of the data and the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-3 and an additional reference. (PDF 11463 kb)
Rights and permissions
About this article
Cite this article
Rücklin, M., Donoghue, P., Johanson, Z. et al. Development of teeth and jaws in the earliest jawed vertebrates. Nature 491, 748–751 (2012). https://doi.org/10.1038/nature11555
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11555
This article is cited by
-
The oldest gnathostome teeth
Nature (2022)
-
A large Middle Devonian eubrachythoracid ‘placoderm’ (Arthrodira) jaw from northern Gondwana
Swiss Journal of Palaeontology (2021)
-
Acanthodian dental development and the origin of gnathostome dentitions
Nature Ecology & Evolution (2021)
-
Current understanding on the Cambrian Explosion: questions and answers
PalZ (2021)
-
The nature of aspidin and the evolutionary origin of bone
Nature Ecology & Evolution (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.