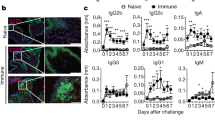
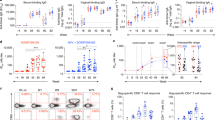
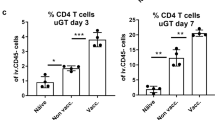
Abstract
Most successful existing vaccines rely on neutralizing antibodies, which may not require specific anatomical localization of B cells. However, efficacious vaccines that rely on T cells for protection have been difficult to develop, as robust systemic memory T-cell responses do not necessarily correlate with host protection1. In peripheral sites, tissue-resident memory T cells provide superior protection compared to circulating memory T cells2,3. Here we describe a simple and non-inflammatory vaccine strategy that enables the establishment of a protective memory T-cell pool within peripheral tissue. The female genital tract, which is a portal of entry for sexually transmitted infections, is an immunologically restrictive tissue that prevents entry of activated T cells in the absence of inflammation or infection4. To overcome this obstacle, we developed a vaccine strategy that we term ‘prime and pull’ to establish local tissue-resident memory T cells at a site of potential viral exposure. This approach relies on two steps: conventional parenteral vaccination to elicit systemic T-cell responses (prime), followed by recruitment of activated T cells by means of topical chemokine application to the restrictive genital tract (pull), where such T cells establish a long-term niche and mediate protective immunity. In mice, prime and pull protocol reduces the spread of infectious herpes simplex virus 2 into the sensory neurons and prevents development of clinical disease. These results reveal a promising vaccination strategy against herpes simplex virus 2, and potentially against other sexually transmitted infections such as human immunodeficiency virus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McElrath, M. J. & Haynes, B. F. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity 33, 542–554 (2010)
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature Immunol. 10, 524–530 (2009)
Jiang, X. et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483, 227–231 (2012)
Nakanishi, Y., Lu, B., Gerard, C. & Iwasaki, A. CD8+ T lymphocyte mobilization to virus-infected tissue requires CD4+ T-cell help. Nature 462, 510–513 (2009)
Koelle, D. M. & Corey, L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu. Rev. Med. 59, 381–395 (2008)
Woodland, D. L. & Kohlmeier, J. E. Migration, maintenance and recall of memory T cells in peripheral tissues. Nature Rev. Immunol. 9, 153–161 (2009)
Masopust, D. et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207, 553–564 (2010)
Klonowski, K. D. et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20, 551–562 (2004)
Groom, J. R. & Luster, A. D. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 89, 207–215 (2011)
Mueller, S. N., Heath, W., McLain, J. D., Carbone, F. R. & Jones, C. M. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol. Cell Biol. 80, 156–163 (2002)
Jones, C. A., Taylor, T. J. & Knipe, D. M. Biological properties of herpes simplex virus 2 replication-defective mutant strains in a murine nasal infection model. Virology 278, 137–150 (2000)
Smith, C. M. et al. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nature Immunol. 5, 1143–1148 (2004)
Kaech, S. M. & Wherry, E. J. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 27, 393–405 (2007)
Weninger, W., Crowley, M. A., Manjunath, N. & von Andrian, U. H. Migratory properties of naive, effector, and memory CD8+ T cells. J. Exp. Med. 194, 953–966 (2001)
Gebhardt, T. et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219 (2011)
Zhu, J. et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nature Med. 15, 886–892 (2009)
Iijima, N. et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J. Exp. Med. 205, 3041–3052 (2008)
Parr, M. B. & Parr, E. L. Intravaginal administration of herpes simplex virus type 2 to mice leads to infection of several neural and extraneural sites. J. Neurovirol. 9, 594–602 (2003)
Mackay, L. K. et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl Acad. Sci. USA 109, 7037–7042 (2012)
Khanna, K. M., Lepisto, A. J. & Hendricks, R. L. Immunity to latent viral infection: many skirmishes but few fatalities. Trends Immunol. 25, 230–234 (2004)
Perkins, N., Nisbet, M. & Thomas, M. Topical imiquimod treatment of aciclovir-resistant herpes simplex disease: case series and literature review. Sex. Transm. Infect. 87, 292–295 (2011)
Gill, N., Davies, E. J. & Ashkar, A. A. The role of Toll-like receptor ligands/agonists in protection against genital HSV-2 infection. Am. J. Reprod. Immunol. 59, 35–43 (2008)
Iwasaki, A. Antiviral immune responses in the genital tract: clues for vaccines. Nature Rev. Immunol. 10, 699–711 (2010)
Gajewski, T. F., Fuertes, M., Spaapen, R., Zheng, Y. & Kline, J. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 23, 286–292 (2011)
Parr, M. B. et al. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab. Invest. 70, 369–380 (1994)
Spang, A. E., Godowski, P. J. & Knipe, D. M. Characterization of herpes simplex virus 2 temperature-sensitive mutants whose lesions map in or near the coding sequences for the major DNA-binding protein. J. Virol. 45, 332–342 (1983)
Malin, S. A., Davis, B. M. & Molliver, D. C. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nature Protocols 2, 152–160 (2007)
Morrison, L. A., Da Costa, X. J. & Knipe, D. M. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 243, 178–187 (1998)
Aljanabi, S. M. & Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 25, 4692–4693 (1997)
Soderberg, K. A., Linehan, M. M., Ruddle, N. H. & Iwasaki, A. MAdCAM-1 expressing sacral lymph node in the lymphotoxin β-deficient mouse provides a site for immune generation following vaginal herpes simplex virus-2 infection. J. Immunol. 173, 1908–1913 (2004)
Acknowledgements
We thank E. Foxman and R. Medzhitov for critical reading of the manuscript, and N. Iijima, H. Dong and B. Yordy for technical support. H.S. is supported by NIAID grant F32AI091024. This work is supported by NIH grants AI054359 and AI062428 to A.I.
Author information
Authors and Affiliations
Contributions
Experiments were conceived and designed by H.S. and A.I. Experiments were performed by H.S. Data were analysed by H.S. and A.I. The paper was written by H.S. and A.I.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-7 and Supplementary Text. (PDF 329 kb)
Rights and permissions
About this article
Cite this article
Shin, H., Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491, 463–467 (2012). https://doi.org/10.1038/nature11522
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11522
This article is cited by
-
A novel “prime and pull” strategy mediated by the combination of two dendritic cell-targeting designs induced protective lung tissue-resident memory T cells against H1N1 influenza virus challenge
Journal of Nanobiotechnology (2023)
-
Olfactory immunology: the missing piece in airway and CNS defence
Nature Reviews Immunology (2023)
-
Establishment of isotype-switched, antigen-specific B cells in multiple mucosal tissues using non-mucosal immunization
npj Vaccines (2023)
-
Assessing the generation of tissue resident memory T cells by vaccines
Nature Reviews Immunology (2023)
-
Localization, tissue biology and T cell state — implications for cancer immunotherapy
Nature Reviews Immunology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.