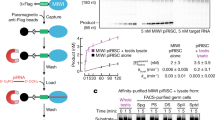
Abstract
PIWI-family proteins and their associated small RNAs (piRNAs) act in an evolutionarily conserved innate immune mechanism to provide essential protection for germ-cell genomes against the activity of mobile genetic elements1. piRNA populations comprise a molecular definition of transposons, which permits them to distinguish transposons from host genes and selectively silence them. piRNAs can be generated in two distinct ways, forming either primary or secondary piRNAs. Primary piRNAs come from discrete genomic loci, termed piRNA clusters, and seem to be derived from long, single-stranded precursors2. The biogenesis of primary piRNAs involves at least two nucleolytic steps. An unknown enzyme cleaves piRNA cluster transcripts to generate monophosphorylated piRNA 5′ ends. piRNA 3′ ends are probably formed by exonucleolytic trimming, after a piRNA precursor is loaded into its PIWI partner1,3. Secondary piRNAs arise during the adaptive ‘ping-pong’ cycle, with their 5′ termini being formed by the activity of PIWIs themselves2,4. A number of proteins have been implicated genetically in primary piRNA biogenesis. One of these, Drosophila melanogaster Zucchini, is a member of the phospholipase-D family of phosphodiesterases, which includes both phospholipases and nucleases5,6,7. Here we produced a dimeric, soluble fragment of the mouse Zucchini homologue (mZuc; also known as PLD6) and show that it possesses single-strand-specific nuclease activity. A crystal structure of mZuc at 1.75 Å resolution indicates greater architectural similarity to phospholipase-D family nucleases than to phospholipases. Together, our data suggest that the Zucchini proteins act in primary piRNA biogenesis as nucleases, perhaps generating the 5′ ends of primary piRNAs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Senti, K. A. & Brennecke, J. The piRNA pathway: a fly’s perspective on the guardian of the genome. Trends Genet. 26, 499–509 (2010)
Brennecke, J. et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128, 1089–1103 (2007)
Kawaoka, S., Izumi, N., Katsuma, S. & Tomari, Y. 3′ end formation of PIWI-interacting RNAs in vitro. Mol. Cell 43, 1015–1022 (2011)
Gunawardane, L. S. et al. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science 315, 1587–1590 (2007)
Haase, A. D. et al. Probing the initiation and effector phases of the somatic piRNA pathway in Drosophila. Genes Dev. 24, 2499–2504 (2010)
Pane, A., Wehr, K. & Schupbach, T. zucchini and squash encode two putative nucleases required for rasiRNA production in the Drosophila germline. Dev. Cell 12, 851–862 (2007)
Schupbach, T. & Wieschaus, E. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics 129, 1119–1136 (1991)
Malone, C. D. et al. Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell 137, 522–535 (2009)
Olivieri, D., Sykora, M. M., Sachidanandam, R., Mechtler, K. & Brennecke, J. An in vivo RNAi assay identifies major genetic and cellular requirements for primary piRNA biogenesis in Drosophila. EMBO J. 29, 3301–3317 (2010)
Selvy, P. E., Lavieri, R. R., Lindsley, C. W. & Brown, H. A. Phospholipase D: enzymology, functionality, and chemical modulation. Chem. Rev. 111, 6064–6119 (2011)
Choi, S. Y. et al. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nature Cell Biol. 8, 1255–1262 (2006)
Huang, H. et al. piRNA-associated germline nuage formation and spermatogenesis require MitoPLD profusogenic mitochondrial-surface lipid signaling. Dev. Cell 20, 376–387 (2011)
Watanabe, T. et al. MITOPLD is a mitochondrial protein essential for nuage formation and piRNA biogenesis in the mouse germline. Dev. Cell 20, 364–375 (2011)
Gonzalvez, F. & Gottlieb, E. Cardiolipin: setting the beat of apoptosis. Apoptosis 12, 877–885 (2007)
Gottlin, E. B., Rudolph, A. E., Zhao, Y., Matthews, H. R. & Dixon, J. E. Catalytic mechanism of the phospholipase D superfamily proceeds via a covalent phosphohistidine intermediate. Proc. Natl Acad. Sci. USA 95, 9202–9207 (1998)
Lackey, D., Walker, G. C., Keng, T. & Linn, S. Characterization of an endonuclease associated with the drug resistance plasmid pKM101. J. Bacteriol. 131, 583–588 (1977)
Pohlman, R. F., Liu, F., Wang, L., More, M. I. & Winans, S. C. Genetic and biochemical analysis of an endonuclease encoded by the IncN plasmid pKM101. Nucleic Acids Res. 21, 4867–4872 (1993)
Stuckey, J. A. & Dixon, J. E. Crystal structure of a phospholipase D family member. Nature Struct. Biol. 6, 278–284 (1999)
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007)
Berg, J. M. & Shi, Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science 271, 1081–1085 (1996)
Lai, W. S., Carballo, E., Thorn, J. M., Kennington, E. A. & Blackshear, P. J. Interactions of CCCH zinc finger proteins with mRNA. Binding of tristetraprolin-related zinc finger proteins to Au-rich elements and destabilization of mRNA. J. Biol. Chem. 275, 17827–17837 (2000)
Kelly, S. M. et al. Recognition of polyadenosine RNA by zinc finger proteins. Proc. Natl Acad. Sci. USA 104, 12306–12311 (2007)
Hurt, J. A. et al. A conserved CCCH-type zinc finger protein regulates mRNA nuclear adenylation and export. J. Cell Biol. 185, 265–277 (2009)
Gao, G., Guo, X. & Goff, S. P. Inhibition of retroviral RNA production by ZAP, a CCCH-type zinc finger protein. Science 297, 1703–1706 (2002)
Van Der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005)
Aravin, A. et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442, 203–207 (2006)
Girard, A., Sachidanandam, R., Hannon, G. J. & Carmell, M. A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442, 199–202 (2006)
Frank, F., Sonenberg, N. & Nagar, B. Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature 465, 818–822 (2010)
Mi, S. et al. Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell 133, 116–127 (2008)
Esnouf, R. M. Further additions to MolScript version 1.4, including reading and contouring of electron-density maps. Acta Crystallogr. D 55, 938–940 (1999)
Bieniossek, C., Richmond, T. J. & Berger, I. MultiBac: multigene baculovirus-based eukaryotic protein complex production. Curr. Protoc. Protein Sci. Chapter 5, Unit 5.20 (2008)
Pozharski, E. V., McWilliams, L. & MacDonald, R. C. Relationship between turbidity of lipid vesicle suspensions and particle size. Anal. Biochem. 291, 158–162 (2001)
Vagin, V. V. et al. A distinct small RNA pathway silences selfish genetic elements in the germline. Science 313, 320–324 (2006)
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26, 795–800 (1993)
Collaborative Computation Project, 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Perrakis, A., Harkiolaki, M., Wilson, K. S. & Lamzin, V. S. ARP/wARP and molecular replacement. Acta Crystallogr. D 57, 1445–1450 (2001)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010)
DeLano, W. L. The PyMOL Molecular Graphics System. (Schrödinger, LLC, 2002)
Holm, L. & Rosenstrom, P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 (2010)
Lindorff-Larsen, K. et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 78, 1950–1958 (2010)
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995)
Acknowledgements
We thank W. Filipowicz, R. MacDonald and members of the G.J.H. and L.J. laboratories for discussions; G. Bencze, K. Rivera and D. Pappin of the Cold Spring Harbor Laboratory proteomics facility, which is funded in part by an National Cancer Institute Cancer Center Support Grant (CA045508), for support with mass spectrometry; and H. Robinson for help at the National Synchrotron Light Source, which is supported by the Department of Energy, Office of Basic Energy Sciences. J.J.I. was supported by the Harvey L. Karp award and by a Ruth L. Kirschstein National Research Service Awards National Institutes of Health (NIH) fellowship F32GM97888. This work was supported by NIH grant R01GM062534. G.J.H. and L.J. are Howard Hughes Medical Institute Investigators.
Author information
Authors and Affiliations
Contributions
L.J., G.J.H., A.D.H. and J.J.I. planned studies and wrote the paper. A.D.H. and J.J.I. performed the experiments, and S.R.K. analysed datasets.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-10, Supplementary Table 1 and additional references. (PDF 9119 kb)
Rights and permissions
About this article
Cite this article
Ipsaro, J., Haase, A., Knott, S. et al. The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature 491, 279–283 (2012). https://doi.org/10.1038/nature11502
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11502
This article is cited by
-
Critical appraisal of the piRNA-PIWI axis in cancer and cancer stem cells
Biomarker Research (2024)
-
Themes and variations on piRNA-guided transposon control
Mobile DNA (2023)
-
The epigenetic regulatory mechanism of PIWI/piRNAs in human cancers
Molecular Cancer (2023)
-
Small RNA sequencing of field Culex mosquitoes identifies patterns of viral infection and the mosquito immune response
Scientific Reports (2023)
-
Delivery of low-density lipoprotein from endocytic carriers to mitochondria supports steroidogenesis
Nature Cell Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.