Abstract
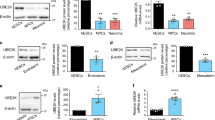
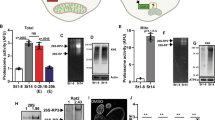
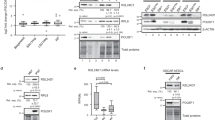
Embryonic stem cells can replicate continuously in the absence of senescence and, therefore, are immortal in culture1,2. Although genome stability is essential for the survival of stem cells, proteome stability may have an equally important role in stem-cell identity and function. Furthermore, with the asymmetric divisions invoked by stem cells, the passage of damaged proteins to daughter cells could potentially destroy the resulting lineage of cells. Therefore, a firm understanding of how stem cells maintain their proteome is of central importance. Here we show that human embryonic stem cells (hESCs) exhibit high proteasome activity that is correlated with increased levels of the 19S proteasome subunit PSMD11 (known as RPN-6 in Caenorhabditis elegans)3,4,5 and a corresponding increased assembly of the 26S/30S proteasome. Ectopic expression of PSMD11 is sufficient to increase proteasome assembly and activity. FOXO4, an insulin/insulin-like growth factor-I (IGF-I) responsive transcription factor associated with long lifespan in invertebrates6,7, regulates proteasome activity by modulating the expression of PSMD11 in hESCs. Proteasome inhibition in hESCs affects the expression of pluripotency markers and the levels of specific markers of the distinct germ layers. Our results suggest a new regulation of proteostasis in hESCs that links longevity and stress resistance in invertebrates to hESC function and identity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Evans, M. J. & Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 (1981)
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998)
Isono, E., Saito, N., Kamata, N., Saeki, Y. & Toh, E. A. Functional analysis of Rpn6p, a lid component of the 26 S proteasome, using temperature-sensitive rpn6 mutants of the yeast Saccharomyces cerevisiae . J. Biol. Chem. 280, 6537–6547 (2005)
Pathare, G. R. et al. The proteasomal subunit Rpn6 is a molecular clamp holding the core and regulatory subcomplexes together. Proc. Natl Acad. Sci. USA 109, 149–154 (2012)
Santamaria, P. G., Finley, D., Ballesta, J. P. & Remacha, M. Rpn6p, a proteasome subunit from Saccharomyces cerevisiae, is essential for the assembly and activity of the 26 S proteasome. J. Biol. Chem. 278, 6687–6695 (2003)
Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R. A. C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993)
Tatar, M. et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292, 107–110 (2001)
Balch, W. E., Morimoto, R. I., Dillin, A. & Kelly, J. W. Adapting proteostasis for disease intervention. Science 319, 916–919 (2008)
Powers, E. T., Morimoto, R. I., Dillin, A., Kelly, J. W. & Balch, W. E. Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 78, 959–991 (2009)
Kisselev, A. F. & Goldberg, A. L. Monitoring activity and inhibition of 26S proteasomes with fluorogenic peptide substrates. Methods Enzymol. 398, 364–378 (2005)
Osafune, K. et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nature Biotechnol. 26, 313–315 (2008)
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007)
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006)
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007)
Hanna, J. H., Saha, K. & Jaenisch, R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell 143, 508–525 (2010)
Panopoulos, A. D., Ruiz, S. & Izpisua Belmonte, J. C. iPSCs: induced back to controversy. Cell Stem Cell 8, 347–348 (2011)
Brennand, K. J. et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225 (2011)
Finley, D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 78, 477–513 (2009)
Köhler, A. et al. The axial channel of the proteasome core particle is gated by the Rpt2 ATPase and controls both substrate entry and product release. Mol. Cell 7, 1143–1152 (2001)
Coux, O., Tanaka, K. & Goldberg, A. L. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 65, 801–847 (1996)
Kops, G. J. P. L. et al. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398, 630–634 (1999)
Matsuzaki, H., Ichino, A., Hayashi, T., Yamamoto, T. & Kikkawa, U. Regulation of intracellular localization and transcriptional activity of FOXO4 by protein kinase B through phosphorylation at the motif sites conserved among the FOXO family. J. Biochem. 138, 485–491 (2005)
Xu, R. H. et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nature Biotechnol. 20, 1261–1264 (2002)
Itoh, M., Kiuru, M., Cairo, M. S. & Christiano, A. M. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc. Natl Acad. Sci. USA 108, 8797–8802 (2011)
Tiscornia, G., Singer, O. & Verma, I. M. Design and cloning of lentiviral vectors expressing small interfering RNAs. Nature Protocols 1, 234–240 (2006)
Schägger, H. Tricine–SDS-PAGE. Nature Protocols 1, 16–22 (2006)
Acknowledgements
We thank W. E. Balch for critical comments on this work. We thank A. Stauffer and V. Modesto for their help with BrdU assays and cell culture, respectively. We thank S. Ruiz for advice on hESC culture and lentiviral infection. This work was supported by the Howard Hughes Medical Institute. D.V. was a recipient of the F.M. Kirby, Inc. Foundation Postdoctoral Scholar Award and Beatriu de Pinós (AGAUR) fellowship. F.H.G. acknowledges the Helmsley Foundation, JPB Foundation, Mathers Foundation, Lookout Fund and California Institute for Regenerative Medicine.
Author information
Authors and Affiliations
Contributions
D.V. and A.D. planned and supervised the project. D.V. performed the experiments, data analysis and interpretation. L.B. performed neural differentiation assays and contributed to other assays. I.M. performed biochemistry experiments and contributed to other assays. M.L. performed cell culturing and trophoblast/fibroblast differentiation. C.M. performed biochemistry experiments and contributed to other assays. D.J. performed proteasome assembly experiments. B.S., L.P. and E.M. generated lentiviral constructs. W.T.B. and F.H.G. contributed with their knowledge of stem-cell biology and neural differentiation, and helped to supervise the project. The manuscript was written by D.V. and A.D. and edited by L.B., I.M., C.M., W.T.B. and F.H.G. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures1-22 and Supplementary Tables 1-8. (PDF 3245 kb)
Supplementary Data
This file contains Supplementary Table 9. (XLS 20 kb)
Rights and permissions
About this article
Cite this article
Vilchez, D., Boyer, L., Morantte, I. et al. Increased proteasome activity in human embryonic stem cells is regulated by PSMD11. Nature 489, 304–308 (2012). https://doi.org/10.1038/nature11468
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11468
This article is cited by
-
Co-expression analysis of transcriptomic data from cancer and healthy specimens reveals rewiring of proteasome genes and an interaction with the XPO1 gene across several tumour types
Translational Medicine Communications (2024)
-
Cold temperature extends longevity and prevents disease-related protein aggregation through PA28γ-induced proteasomes
Nature Aging (2023)
-
Artificial induction of circadian rhythm by combining exogenous BMAL1 expression and polycomb repressive complex 2 inhibition in human induced pluripotent stem cells
Cellular and Molecular Life Sciences (2023)
-
The fiber diameter traits of Tibetan cashmere goats are governed by the inherent differences in stress, hypoxic, and metabolic adaptations: an integrative study of proteome and transcriptome
BMC Genomics (2022)
-
Fbxw11 impairs the repopulation capacity of hematopoietic stem/progenitor cells
Stem Cell Research & Therapy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.