Abstract
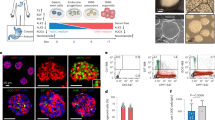
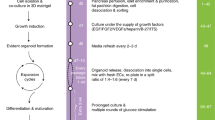
One goal of regenerative medicine, to use stem cells to replace cells lost by injury or disease, depends on producing an excess of the relevant cell for study or transplantation. To this end, the stepwise differentiation of stem cells into specialized derivatives has been successful for some cell types1,2,3, but a major problem remains the inefficient conversion of cells from one stage of differentiation to the next. If specialized cells are to be produced in large numbers it will be necessary to expand progenitor cells, without differentiation, at some steps of the process. Using the pancreatic lineage as a model for embryonic-stem-cell differentiation, we demonstrate that this is a solvable problem. Co-culture with organ-matched mesenchyme permits proliferation and self-renewal of progenitors, without differentiation, and enables an expansion of more than a million-fold for human endodermal cells with full retention of their developmental potential. This effect is specific both to the mesenchymal cell and to the progenitor being amplified. Progenitors that have been serially expanded on mesenchyme give rise to glucose-sensing, insulin-secreting cells when transplanted in vivo. Theoretically, the identification of stage-specific renewal signals can be incorporated into any scheme for the efficient production of large numbers of differentiated cells from stem cells and may therefore have wide application in regenerative biology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kroon, E. et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature Biotechnol. 26, 443–452 (2008)
Grigoriadis, A. E. et al. Directed differentiation of hematopoietic precursors and functional osteoclasts from human ES and iPS cells. Blood 115, 2769–2776 (2010)
Perrier, A. L. et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl Acad. Sci. USA 101, 12543–12548 (2004)
Moore, K. A. & Lemischka, I. R. Stem cells and their niches. Science 311, 1880–1885 (2006)
Yamashita, Y. M. & Fuller, M. T. Asymmetric stem cell division and function of the niche in the Drosophila male germ line. Int. J. Hematol. 82, 377–380 (2005)
Lammert, E., Cleaver, O. & Melton, D. Induction of pancreatic differentiation by signals from blood vessels. Science 294, 564–567 (2001)
Golosow, N. & Grobstein, C. Epitheliomesenchymal interaction in pancreatic morphogenesis. Dev. Biol. 4, 242–255 (1962)
Wessells, N. K. & Cohen, J. H. Early pancreas organogenesis: morphogenesis, tissue interactions, and mass effects. Dev. Biol. 15, 237–270 (1967)
Kanai-Azuma, M. et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 129, 2367–2379 (2002)
Lee, C. S., Perreault, N., Brestelli, J. E. & Kaestner, K. H. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 16, 1488–1497 (2002)
Chambers, I. & Smith, A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene 23, 7150–7160 (2004)
Becker, A. J., Mc, C. E. & Till, J. E. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 197, 452–454 (1963)
James, R. & Kazenwadel, J. Homeobox gene expression in the intestinal epithelium of adult mice. J. Biol. Chem. 266, 3246–3251 (1991)
Que, J. et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134, 2521–2531 (2007)
Thompson, D. A. W. On Growth and Form (Cambridge Univ. Press, 1917)
Borowiak, M. et al. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell 4, 348–358 (2009)
Kim, I., Saunders, T. L. & Morrison, S. J. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130, 470–483 (2007)
Lengner, C. J. et al. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell 1, 403–415 (2007)
Rezania, A. et al. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes 60, 239–247 (2007)
D’Amour, K. A. et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nature Biotechnol. 23, 1534–1541 (2005)
Chen, S. et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nature Chem. Biol. 5, 258–265 (2009)
Cowan, C. A. et al. Derivation of embryonic stem-cell lines from human blastocysts. N. Engl. J. Med. 350, 1353–1356 (2004)
Bosco, D., Meda, P., Halban, P. A. & Rouiller, D. G. Importance of cell-matrix interactions in rat islet beta-cell secretion in vitro: role of α6β1 integrin. Diabetes 49, 233–243 (2000)
Arbiser, J. L. et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc. Natl Acad. Sci. USA 94, 861–866 (1997)
Montesano, R. et al. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell 62, 435–445 (1990)
Acknowledgements
We thank S. Morrison for providing the Sox17–GFP reporter mouse ESC line and K. Kaestner for the Ngn3–GFP knock-in mouse ESCs. We also thank J. LaVecchio, G. Buruzula and B. Tilton for support for cell sorting, A. Kweudjeu for help with gene-expression experiments and human ESC culture, and C. Xie, C. Balatbat and K. Koszka for technical assistance. We are grateful to A. Tward and D. Cohen for critical reading of the manuscript. We thank J. Annes for assistance in obtaining human tissue samples and acknowledge the use of human tissues provided by the National Disease Research Interchange (NDRI), with support from National Institutes of Health grants 5 U42 RR006042-20 and K08 DK084206. J.B.S. is supported by the Howard Hughes Medical Institute. M.B. was supported by a grant from The Leona M. and Harry B. Helmsley Charitable Trust. D.A.M. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
Author Contributions J.B.S., M.B., and D.A.M. conceived and designed the research. J.B.S. and M.B. carried out the experiments, and J.B.S., M.B. and D.A.M. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-11, Supplementary Tables 1-2 and additional references. (PDF 2806 kb)
Rights and permissions
About this article
Cite this article
Sneddon, J., Borowiak, M. & Melton, D. Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature 491, 765–768 (2012). https://doi.org/10.1038/nature11463
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11463
This article is cited by
-
Human pancreatic microenvironment promotes β-cell differentiation via non-canonical WNT5A/JNK and BMP signaling
Nature Communications (2022)
-
Improved Differentiation of hESC-Derived Pancreatic Progenitors by Using Human Fetal Pancreatic Mesenchymal Cells in a Micro‐scalable Three-Dimensional Co-culture System
Stem Cell Reviews and Reports (2022)
-
Engineering islets from stem cells for advanced therapies of diabetes
Nature Reviews Drug Discovery (2021)
-
Generation of insulin-producing pancreatic β cells from multiple human stem cell lines
Nature Protocols (2021)
-
The promise of stem cell-derived islet replacement therapy
Diabetologia (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.