Abstract
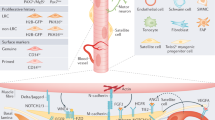
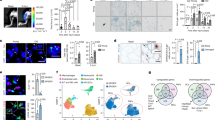
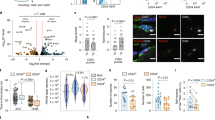
The niche is a conserved regulator of stem cell quiescence and function. During ageing, stem cell function declines. To what extent and by what means age-related changes within the niche contribute to this phenomenon are unknown. Here we demonstrate that the aged muscle stem cell niche, the muscle fibre, expresses Fgf2 under homeostatic conditions, driving a subset of satellite cells to break quiescence and lose their self-renewing capacity. We show in mice that relatively dormant aged satellite cells robustly express sprouty 1 (Spry1), an inhibitor of fibroblast growth factor (FGF) signalling. Increasing FGF signalling in aged satellite cells under homeostatic conditions by removing Spry1 results in the loss of quiescence, satellite cell depletion and diminished regenerative capacity. Conversely, reducing niche-derived FGF activity through inhibition of Fgfr1 signalling or overexpression of Spry1 in satellite cells prevents their depletion. These experiments identify an age-dependent change in the stem cell niche that directly influences stem cell quiescence and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sambasivan, R. et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647–3656 (2011)
Collins, C. A. et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122, 289–301 (2005)
Lepper, C., Partridge, T. A. & Fan, C. M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639–3646 (2011)
Sacco, A., Doyonnas, R., Kraft, P., Vitorovic, S. & Blau, H. M. Self-renewal and expansion of single transplanted muscle stem cells. Nature 456, 502–506 (2008)
Murphy, M. M., Lawson, J. A., Mathew, S. J., Hutcheson, D. A. & Kardon, G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138, 3625–3637 (2011)
Shea, K. L. et al. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell 6, 117–129 (2010)
Shavlakadze, T., McGeachie, J. & Grounds, M. D. Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology 11, 363–376 (2010)
Carlson, B. M. & Faulkner, J. A. Muscle transplantation between young and old rats: age of host determines recovery. Am. J. Physiol. 256, C1262–C1266 (1989)
Brack, A. S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 (2007)
Collins, C. A., Zammit, P. S., Ruiz, A. P., Morgan, J. E. & Partridge, T. A. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells 25, 885–894 (2007)
Conboy, I. M., Conboy, M. J., Smythe, G. M. & Rando, T. A. Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575–1577 (2003)
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005)
Shefer, G., Van de Mark, D. P., Richardson, J. B. & Yablonka-Reuveni, Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev. Biol. 294, 50–66 (2006)
Brack, A. S., Bildsoe, H. & Hughes, S. M. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J. Cell Sci. 118, 4813–4821 (2005)
Carlson, M. E., Hsu, M. & Conboy, I. M. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature 454, 528–532 (2008)
Pan, L. et al. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell 1, 458–469 (2007)
Boyle, M., Wong, C., Rocha, M. & Jones, D. L. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell 1, 470–478 (2007)
Voog, J. & Jones, D. L. Stem cells and the niche: a dynamic duo. Cell Stem Cell 6, 103–115 (2010)
Morrison, S. J. & Spradling, A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008)
Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493–495 (1961)
Bischoff, R. Interaction between satellite cells and skeletal muscle fibers. Development 109, 943–952 (1990)
Orford, K. W. & Scadden, D. T. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nature Rev. Genet. 9, 115–128 (2008)
Foudi, A. et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nature Biotechnol. 27, 84–90 (2009)
Zammit, P. S. et al. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 166, 347–357 (2004)
Olguin, H. C. & Olwin, B. B. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev. Biol. 275, 375–388 (2004)
Rocheteau, P., Gayraud-Morel, B., Siegl-Cachedenier, I., Blasco, M. A. & Tajbakhsh, S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell 148, 112–125 (2012)
Yablonka-Reuveni, Z. & Rivera, A. J. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev. Biol. 164, 588–603 (1994)
Sheehan, S. M. & Allen, R. E. Skeletal muscle satellite cell proliferation in response to members of the fibroblast growth factor family and hepatocyte growth factor. J. Cell. Physiol. 181, 499–506 (1999)
Bischoff, R. A satellite cell mitogen from crushed adult muscle. Dev. Biol. 115, 140–147 (1986)
Yoshida, N., Yoshida, S., Koishi, K., Masuda, K. & Nabeshima, Y. Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf-5 generates ‘reserve cells’. J. Cell Sci. 111, 769–779 (1998)
Hacohen, N., Kramer, S., Sutherland, D., Hiromi, Y. & Krasnow, M. A. sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell 92, 253–263 (1998)
Kim, H. J. & Bar-Sagi, D. Modulation of signalling by Sprouty: a developing story. Nature Rev. Mol. Cell Biol. 5, 441–450 (2004)
Gross, I., Bassit, B., Benezra, M. & Licht, J. D. Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. J. Biol. Chem. 276, 46460–46468 (2001)
Thum, T. et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456, 980–984 (2008)
Yang, X. et al. Overexpression of Spry1 in chondrocytes causes attenuated FGFR ubiquitination and sustained ERK activation resulting in chondrodysplasia. Dev. Biol. 321, 64–76 (2008)
Wilson, A. et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 (2008)
Floss, T., Arnold, H. H. & Braun, T. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 11, 2040–2051 (1997)
Lefaucheur, J. P. & Sebille, A. Muscle regeneration following injury can be modified in vivo by immune neutralization of basic fibroblast growth factor, transforming growth factor beta 1 or insulin-like growth factor I. J. Neuroimmunol. 57, 85–91 (1995)
Conboy, I. M. & Rando, T. A. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell 3, 397–409 (2002)
Morrison, S. J., Wandycz, A. M., Akashi, K., Globerson, A. & Weissman, I. L. The aging of hematopoietic stem cells. Nature Med. 2, 1011–1016 (1996)
Sudo, K., Ema, H., Morita, Y. & Nakauchi, H. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 192, 1273–1280 (2000)
Ono, Y. et al. Slow-dividing satellite cells retain long-term self-renewal ability in adult muscle. J. Cell Sci. 125, 1309–1319 (2012)
Kuang, S., Kuroda, K., Le Grand, F. & Rudnicki, M. A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999–1010 (2007)
Bjornson, C. R. et al. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells 30, 232–242 (2012)
Cheung, T. H. et al. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature 482, 524–528 (2012)
Mourikis, P. et al. A critical requirement for Notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells 30, 243–252 (2011)
Lagha, M. et al. Pax3 regulation of FGF signaling affects the progression of embryonic progenitor cells into the myogenic program. Genes Dev. 22, 1828–1837 (2008)
Groves, J. A., Hammond, C. L. & Hughes, S. M. Fgf8 drives myogenic progression of a novel lateral fast muscle fibre population in zebrafish. Development 132, 4211–4222 (2005)
Flanagan-Steet, H., Hannon, K., McAvoy, M. J., Hullinger, R. & Olwin, B. B. Loss of FGF receptor 1 signaling reduces skeletal muscle mass and disrupts myofiber organization in the developing limb. Dev. Biol. 218, 21–37 (2000)
Kudla, A. J. et al. The FGF receptor-1 tyrosine kinase domain regulates myogenesis but is not sufficient to stimulate proliferation. J. Cell Biol. 142, 241–250 (1998)
Basson, M. A. et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev. Cell 8, 229–239 (2005)
Xu, X., Qiao, W., Li, C. & Deng, C. X. Generation of Fgfr1 conditional knockout mice. Genesis 32, 85–86 (2002)
Nishijo, K. et al. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J. 23, 2681–2690 (2009)
Buono, M., Visigalli, I., Bergamasco, R., Biffi, A. & Cosma, M. P. Sulfatase modifying factor 1-mediated fibroblast growth factor signaling primes hematopoietic multilineage development. J. Exp. Med. 207, 1647–1660 (2010)
Umemori, H., Linhoff, M. W., Ornitz, D. M. & Sanes, J. R. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell 118, 257–270 (2004)
Acknowledgements
We thank H. Hock, K. Hochedlinger and R. Friesel for the generous provision of reagents, and G. Estrada for technical assistance. We are also grateful to L. Prickett-Rice, K. Folz-Donahue and M. Weglarz for cell sorting. This work was supported by MGH start-up funds, Harvard Stem Cell Institute grants and NIH grants (R01 AR060868, R01 AR061002) (A.S.B.); a Wellcome Trust grant (WT091475) (M.A.B.); and an MGH ECOR Postdoctoral Fellow Award (J.V.C.) and a BBSRC Doctoral Training Award (BB/F017626/1) (K.M.J.).
Author information
Authors and Affiliations
Contributions
J.V.C. designed and performed experiments, analysed data, interpreted results and wrote the manuscript. K.M.J. performed experiments, analysed data and edited the manuscript. M.A.B. conceived the project, designed experiments, interpreted results, and edited the manuscript. A.S.B. conceived the project, designed experiments, interpreted results, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-11. (PDF 13124 kb)
Rights and permissions
About this article
Cite this article
Chakkalakal, J., Jones, K., Basson, M. et al. The aged niche disrupts muscle stem cell quiescence. Nature 490, 355–360 (2012). https://doi.org/10.1038/nature11438
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11438
This article is cited by
-
Neurofibromin 1 controls metabolic balance and Notch-dependent quiescence of murine juvenile myogenic progenitors
Nature Communications (2024)
-
Insight into muscle stem cell regeneration and mechanobiology
Stem Cell Research & Therapy (2023)
-
Prim-O-glucosylcimifugin ameliorates aging-impaired endogenous tendon regeneration by rejuvenating senescent tendon stem/progenitor cells
Bone Research (2023)
-
The meaning of adaptation in aging: insights from cellular senescence, epigenetic clocks and stem cell alterations
Nature Aging (2023)
-
Ageing and rejuvenation of tissue stem cells and their niches
Nature Reviews Molecular Cell Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.