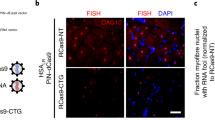
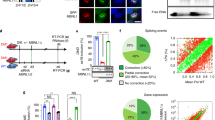
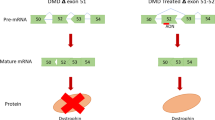
Abstract
Antisense oligonucleotides (ASOs) hold promise for gene-specific knockdown in diseases that involve RNA or protein gain-of-function effects. In the hereditary degenerative disease myotonic dystrophy type 1 (DM1), transcripts from the mutant allele contain an expanded CUG repeat1,2,3 and are retained in the nucleus4,5. The mutant RNA exerts a toxic gain-of-function effect6, making it an appropriate target for therapeutic ASOs. However, despite improvements in ASO chemistry and design, systemic use of ASOs is limited because uptake in many tissues, including skeletal and cardiac muscle, is not sufficient to silence target messenger RNAs7,8. Here we show that nuclear-retained transcripts containing expanded CUG (CUGexp) repeats are unusually sensitive to antisense silencing. In a transgenic mouse model of DM1, systemic administration of ASOs caused a rapid knockdown of CUGexp RNA in skeletal muscle, correcting the physiological, histopathologic and transcriptomic features of the disease. The effect was sustained for up to 1 year after treatment was discontinued. Systemically administered ASOs were also effective for muscle knockdown of Malat1, a long non-coding RNA (lncRNA) that is retained in the nucleus9. These results provide a general strategy to correct RNA gain-of-function effects and to modulate the expression of expanded repeats, lncRNAs and other transcripts with prolonged nuclear residence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Harley, H. G. et al. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature 355, 545–546 (1992)
Buxton, J. et al. Detection of an unstable fragment of DNA specific to individuals with myotonic dystrophy. Nature 355, 547–548 (1992)
Brook, J. D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808 (1992)
Taneja, K. L., McCurrach, M., Schalling, M., Housman, D. & Singer, R. H. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J. Cell Biol. 128, 995–1002 (1995)
Davis, B. M., McCurrach, M. E., Taneja, K. L., Singer, R. H. & Housman, D. E. Expansion of a CUG trinucleotide repeat in the 3′ untranslated region of myotonic dystrophy protein kinase transcripts results in nuclear retention of transcripts. Proc. Natl Acad. Sci. USA 94, 7388–7393 (1997)
Mankodi, A. et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 289, 1769–1773 (2000)
Bennett, C. F. & Swayze, E. E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 50, 259–293 (2010)
Geary, R. S. et al. Pharmacokinetics of a tumor necrosis factor-alpha phosphorothioate 2′-O-(2-methoxyethyl) modified antisense oligonucleotide: comparison across species. Drug Metab. Dispos. 31, 1419–1428 (2003)
Wilusz, J. E., Freier, S. M. & Spector, D. L. 3′ end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell 135, 919–932 (2008)
Lorenz, P., Misteli, T., Baker, B. F., Bennett, C. F. & Spector, D. L. Nucleocytoplasmic shuttling: a novel in vivo property of antisense phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 28, 582–592 (2000)
Vickers, T. A. et al. Efficient reduction of target RNAs by small interfering RNA and RNase H-dependent antisense agents. A comparative analysis. J. Biol. Chem. 278, 7108–7118 (2003)
Prasanth, K. V. et al. Regulating gene expression through RNA nuclear retention. Cell 123, 249–263 (2005)
Wu, H. et al. Determination of the role of the human RNase H1 in the pharmacology of DNA-like antisense drugs. J. Biol. Chem. 279, 17181–17189 (2004)
Suzuki, Y. et al. An upstream open reading frame and the context of the two AUG codons affect the abundance of mitochondrial and nuclear RNase H1. Mol. Cell. Biol. 30, 5123–5134 (2010)
Lin, X. et al. Failure of MBNL1-dependent post-natal splicing transitions in myotonic dystrophy. Hum. Mol. Genet. 15, 2087–2097 (2006)
Osborne, R. J. et al. Transcriptional and post-transcriptional impact of toxic RNA in myotonic dystrophy. Hum. Mol. Genet. 18, 1471–1481 (2009)
Kanadia, R. N. et al. A muscleblind knockout model for myotonic dystrophy. Science 302, 1978–1980 (2003)
Wheeler, T. M. et al. Reversal of RNA dominance by displacement of protein sequestered on triplet repeat RNA. Science 325, 336–339 (2009)
Hasselblatt, P., Hockenjos, B., Thoma, C., Blum, H. E. & Offensperger, W. B. Translation of stable hepadnaviral mRNA cleavage fragments induced by the action of phosphorothioate-modified antisense oligodeoxynucleotides. Nucleic Acids Res. 33, 114–125 (2005)
Napierala, M. & Krzyzosiak, W. J. CUG repeats present in myotonin kinase RNA form metastable “slippery” hairpins. J. Biol. Chem. 272, 31079–31085 (1997)
Miller, J. W. et al. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J. 19, 4439–4448 (2000)
Mankodi, A. et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol. Cell 10, 35–44 (2002)
Du, H. et al. Aberrant alternative splicing and extracellular matrix gene expression in mouse models of myotonic dystrophy. Nature Struct. Mol. Biol. 17, 187–193 (2010)
Alter, J. et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nature Med. 12, 175–177 (2006)
Pontius, B. W. & Berg, P. Rapid assembly and disassembly of complementary DNA strands through an equilibrium intermediate state mediated by A1 hnRNP protein. J. Biol. Chem. 267, 13815–13818 (1992)
Li, L. B. & Bonini, N. M. Roles of trinucleotide-repeat RNA in neurological disease and degeneration. Trends Neurosci. 33, 292–298 (2010)
DeJesus-Hernandez, M. et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256 (2011)
Mulders, S. A. et al. Triplet-repeat oligonucleotide-mediated reversal of RNA toxicity in myotonic dystrophy. Proc. Natl Acad. Sci. USA 106, 13915–13920 (2009)
Lee, J. E., Bennett, C. F. & Cooper, T. A. RNase H-mediated degradation of toxic RNA in myotonic dystrophy type 1. Proc. Natl Acad. Sci. USA 109, 4221–4226 (2012)
Wapinski, O. & Chang, H. Y. Long noncoding RNAs and human disease. Trends Cell Biol. 21, 354–361 (2011)
Cheruvallath, Z. S., Kumar, R. K., Rentel, C., Cole, D. L. & Ravikumar, V. T. Solid phase synthesis of phosphorothioate oligonucleotides utilizing diethyldithiocarbonate disulfide (DDD) as an efficient sulfur transfer reagent. Nucleosides Nucleotides Nucleic Acids 22, 461–468 (2003)
Seznec, H. et al. Transgenic mice carrying large human genomic sequences with expanded CTG repeat mimic closely the DM CTG repeat intergenerational and somatic instability. Hum. Mol. Genet. 9, 1185–1194 (2000)
Nakamori, M., Gourdon, G. & Thornton, C. A. Stabilization of expanded (CTG)*(CAG) repeats by antisense oligonucleotides. Mol. Ther. 19, 2222–2227 (2011)
Seznec, H. et al. Mice transgenic for the human myotonic dystrophy region with expanded CTG repeats display muscular and brain abnormalities. Hum. Mol. Genet. 10, 2717–2726 (2001)
Gomes-Pereira, M. et al. CTG trinucleotide repeat “big jumps”: large expansions, small mice. PLoS Genet. 3, e52 (2007)
Wheeler, T. M., Lueck, J. D., Swanson, M. S., Dirksen, R. T. & Thornton, C. A. Correction of ClC-1 splicing eliminates chloride channelopathy and myotonia in mouse models of myotonic dystrophy. J. Clin. Invest. 117, 3952–3957 (2007)
Koller, E. et al. Mechanisms of single-stranded phosphorothioate modified antisense oligonucleotide accumulation in hepatocytes. Nucleic Acids Res. 39, 4795–4807 (2011)
Ihaka, R. & Gentleman, R. R. A language for data analysis and graphics. J. Comput. Graph. Stat. 5, 299–314 (1996)
Raychaudhuri, S., Stuart, J. M. & Altman, R. B. Principal components analysis to summarize microarray experiments: application to sporulation time series. Pac. Symp. Biocomput. 2000, 455–466 (2000)
Ringnér, M. What is principal component analysis? Nature Biotechnol. 26, 303–304 (2008)
Briguet, A., Courdier-Fruh, I., Foster, M., Meier, T. & Magyar, J. P. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul. Disord. 14, 675–682 (2004)
Leeds, J. M., Graham, M. J., Truong, L. & Cummins, L. L. Quantitation of phosphorothioate oligonucleotides in human plasma. Anal. Biochem. 235, 36–43 (1996)
Geary, R. S., Matson, J. & Levin, A. A. A nonradioisotope biomedical assay for intact oligonucleotide and its chain-shortened metabolites used for determination of exposure and elimination half-life of antisense drugs in tissue. Anal. Biochem. 274, 241–248 (1999)
Acknowledgements
The work was carried out at the Wellstone Muscular Dystrophy Cooperative Research Center and Center for RNA Biology at the University of Rochester, with support from the US National Institutes of Health (NIH) (grants AR049077, U54NS48843, AR/NS48143, K08NS064293 and U01NS072323), the Saunders Family Neuromuscular Research Fund, Run America and a fellowship (to M.N.) from the Muscular Dystrophy Association and Uehara Memorial Foundation. The authors thank G. Gourdon for providing DM328XL mice, M. Sabripour for assistance with principal components analysis and L. Richardson and S. Leistman for technical assistance.
Author information
Authors and Affiliations
Contributions
Author Contributions T.M.W., A.J.L, S.K.P., A.R.M., M.N., S.H.C., B.M.W., C.F.B. and C.A.T. participated in the planning, design and interpretation of experiments. T.M.W., A.J.L., S.K.P., A.R.M and M.N. carried out experiments. T.M.W. and C.A.T. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.K.P., A.R.M. and C.F.B. are employees of Isis Pharmaceuticals, which develops antisense drugs. A.J.L., S.H.C. and B.M.W. are employees of Genzyme Corporation, which develops biological therapeutics. C.A.T. and T.M.W. received research support from Isis Pharmaceuticals. Isis Pharmaceuticals has applied for patents related to this work.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-18, Supplementary Tables 1-3 and Supplementary References. (PDF 18481 kb)
Rights and permissions
About this article
Cite this article
Wheeler, T., Leger, A., Pandey, S. et al. Targeting nuclear RNA for in vivo correction of myotonic dystrophy. Nature 488, 111–115 (2012). https://doi.org/10.1038/nature11362
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11362
This article is cited by
-
Correction of Clcn1 alternative splicing reverses muscle fiber type transition in mice with myotonic dystrophy
Nature Communications (2023)
-
A novel long noncoding RNA SP100-AS1 induces radioresistance of colorectal cancer via sponging miR-622 and stabilizing ATG3
Cell Death & Differentiation (2023)
-
Non-coding RNAs in human health and disease: potential function as biomarkers and therapeutic targets
Functional & Integrative Genomics (2023)
-
CircARID1A binds to IGF2BP3 in gastric cancer and promotes cancer proliferation by forming a circARID1A-IGF2BP3-SLC7A5 RNA–protein ternary complex
Journal of Experimental & Clinical Cancer Research (2022)
-
Antisense oligonucleotides as a potential treatment for brain deficits observed in myotonic dystrophy type 1
Gene Therapy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.