Abstract
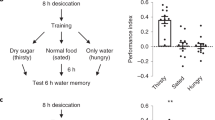
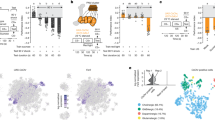
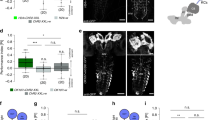
Animals approach stimuli that predict a pleasant outcome1. After the paired presentation of an odour and a reward, Drosophila melanogaster can develop a conditioned approach towards that odour2,3. Despite recent advances in understanding the neural circuits for associative memory and appetitive motivation4, the cellular mechanisms for reward processing in the fly brain are unknown. Here we show that a group of dopamine neurons in the protocerebral anterior medial (PAM) cluster signals sugar reward by transient activation and inactivation of target neurons in intact behaving flies. These dopamine neurons are selectively required for the reinforcing property of, but not a reflexive response to, the sugar stimulus. In vivo calcium imaging revealed that these neurons are activated by sugar ingestion and the activation is increased on starvation. The output sites of the PAM neurons are mainly localized to the medial lobes of the mushroom bodies (MBs), where appetitive olfactory associative memory is formed5,6. We therefore propose that the PAM cluster neurons endow a positive predictive value to the odour in the MBs. Dopamine in insects is known to mediate aversive reinforcement signals5,7,8,9,10,11. Our results highlight the cellular specificity underlying the various roles of dopamine and the importance of spatially segregated local circuits within the MBs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schultz, W. Behavioral theories and the neurophysiology of reward. Annu. Rev. Psychol. 57, 87–115 (2006)
Tempel, B. L., Bonini, N., Dawson, D. R. & Quinn, W. G. Reward learning in normal and mutant Drosophila. Proc. Natl Acad. Sci. USA 80, 1482–1486 (1983)
Kaun, K. R., Azanchi, R., Maung, Z., Hirsh, J. & Heberlein, U. A Drosophila model for alcohol reward. Nature Neurosci. 14, 612–619 (2011)
Waddell, S. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 33, 457–464 (2010)
Schwaerzel, M. et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10495–10502 (2003)
Trannoy, S., Redt-Clouet, C., Dura, J. M. & Preat, T. Parallel processing of appetitive short- and long-term memories in Drosophila. Curr. Biol. 21, 1647–1653 (2011)
Riemensperger, T., Völler, T., Stock, P., Buchner, E. & Fiala, A. Punishment prediction by dopaminergic neurons in Drosophila. Curr. Biol. 15, 1953–1960 (2005)
Claridge-Chang, A. et al. Writing memories with light-addressable reinforcement circuitry. Cell 139, 405–415 (2009)
Mao, Z. & Davis, R. L. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front. Neural Circuits 3, 5 (2009)
Aso, Y. et al. Specific dopaminergic neurons for the formation of labile aversive memory. Curr. Biol. 20, 1445–1451 (2010)
Mizunami, M. & Matsumoto, Y. Roles of aminergic neurons in formation and recall of associative memory in crickets. Front. Behav. Neurosci. 4, 172 (2010)
Hammer, M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature 366, 59–63 (1993)
Schroll, C. et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 16, 1741–1747 (2006)
Yarali, A. & Gerber, B. A neurogenetic dissociation between punishment-, reward-, and relief-learning in Drosophila. Front. Behav. Neurosci. 4, 189 (2010)
Krashes, M. J. et al. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139, 416–427 (2009)
Kim, Y. C., Lee, H. G. & Han, K. A. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J. Neurosci. 27, 7640–7647 (2007)
Selcho, M., Pauls, D., Han, K. A., Stocker, R. F. & Thum, A. S. The role of dopamine in Drosophila larval classical olfactory conditioning. PLoS ONE 4, e5897 (2009)
Hamada, F. N. et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220 (2008)
Sitaraman, D. et al. Serotonin is necessary for place memory in Drosophila. Proc. Natl Acad. Sci. USA 105, 5579–5584 (2008)
Kitamoto, T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 47, 81–92 (2001)
Pfeiffer, B. D. et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl Acad. Sci. USA 105, 9715–9720 (2008)
Monastirioti, M., Linn, C. E., Jr & White, K. Characterization of Drosophila tyramine β-hydroxylase gene and isolation of mutant flies lacking octopamine. J. Neurosci. 16, 3900–3911 (1996)
Busch, S., Selcho, M., Ito, K. & Tanimoto, H. A map of octopaminergic neurons in the Drosophila brain. J. Comp. Neurol. 513, 643–667 (2009)
Tanaka, N. K., Tanimoto, H. & Ito, K. Neuronal assemblies of the Drosophila mushroom body. J. Comp. Neurol. 508, 711–755 (2008)
van Swinderen, B. & Andretic, R. Dopamine in Drosophila: setting arousal thresholds in a miniature brain. Proc. R. Soc. B 278, 906–913 (2011)
Bromberg-Martin, E. S., Matsumoto, M. & Hikosaka, O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834 (2010)
Pfeiffer, B. D. et al. Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735–755 (2010)
Schnaitmann, C., Vogt, K., Triphan, T. & Tanimoto, H. Appetitive and aversive visual learning in freely moving Drosophila. Front. Behav. Neurosci. 4, 10 (2010)
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods 6, 875–881 (2009)
Séjourné, J. et al. Mushroom body efferent neurons responsible for aversive olfactory memory retrieval in Drosophila. Nature Neurosci. 14, 903–910 (2011)
Kume, K. et al. Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 25, 7377–7384 (2005)
Friggi-Grelin, F. et al. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 54, 618–627 (2003)
Li, H., Chaney, S., Roberts, I. J., Forte, M. & Hirsh, J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr. Biol. 10, 211–214 (2000)
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999)
Robinson, I. M., Ranjan, R. & Schwarz, T. L. Synaptotagmins I and IV promote transmitter release independently of Ca2+ binding in the C2A domain. Nature 418, 336–340 (2002)
Cole, S. H. et al. Two functional but non-complementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J. Biol. Chem. 280, 14948–14955 (2005)
Marella, S. et al. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron 49, 285–295 (2006)
Acknowledgements
We thank L. Bräcker, M. Feind, C. Murphy, C. Schnaitmann and T. Templier for technical assistance and experiments that inspired this study; P. Garrity, the Kyoto Drosophila Genetic Resource Center and the Bloomington Stock Center for fly stocks; and Y.Y. Ma, Z. Q. Meng, R. Menzel, A. Thum, S. Waddell and the members of the Tanimoto laboratory for discussion and/or critical reading of the manuscript. C.L., Y.A., N.Y. and P.-Y.P. were sponsored by a Chinese–European doctoral training program from Max-Planck-Gesellschaft and the Chinese Academy of Sciences, the Deutscher Akademischer Austausch Dienst, the Alexander von Humboldt Foundation, and the Région Île-de-France, respectively. This work was supported by the Agence Nationale pour la Recherche (T.P.), the Howard Hughes Medical Institute (G.M.R.), Bernstein Focus Learning from the Bundesministerium für Bildung und Forschung and the Max-Planck-Gesellschaft (H.T.).
Author information
Authors and Affiliations
Contributions
C.L., N.Y., Y.A. and H.T. designed and C.L. and N.Y. performed all the behavioural experiments in this study. P.Y.P., T.P. and H.T. designed in vivo imaging experiments, and P.Y.P. and T.P. devised a new gustatory stimulation method. P.Y.P. performed imaging experiments and analysed the data. B.D.P. and G.M.R. designed and generated the new transgenic flies (GAL4, GAL80, LexA and LexAop2-dTrpA1 lines). Y.A. and H.T. identified R58E02 by using a database of GAL4 expression patterns created by G.M.R. and the Janelia Farm Fly Light Project Team. A.B.F. and I.S. performed immunohistochemistry, and C.L., A.B.F. and H.T. analysed the microscopic data. C.L. and H.T. made the figures and wrote the paper with the help of all the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9 and full legends for Supplementary Movies 1-5. (PDF 2556 kb)
Supplementary Movie 1
This file contains a movie showing the expression pattern of R58E02-GAL4 in the brain. (AVI 5628 kb)
Supplementary Movie 2
This file contains a movie showing the expression pattern of DDC-GAL4 in the central brain. (AVI 4362 kb)
Supplementary Movie 3
This file contains a movie showing the expression pattern of DDC-GAL4 with R58E02-GAL80 in the central brain. (AVI 3834 kb)
Supplementary Movie 4
This file contains a movie showing differential labelling of NP5272-GAL4 and R58E02-LexA in the cell body region of the PAM cluster. (AVI 116 kb)
Supplementary Movie 5
This file contains a movie showing differential labelling of TH-GAL4 and R58E02-LexA in the MB. (AVI 3819 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Plaçais, PY., Yamagata, N. et al. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 488, 512–516 (2012). https://doi.org/10.1038/nature11304
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11304
This article is cited by
-
Multisensory learning binds neurons into a cross-modal memory engram
Nature (2023)
-
Circadian clock disruption promotes the degeneration of dopaminergic neurons in male Drosophila
Nature Communications (2023)
-
Dopaminergic systems create reward seeking despite adverse consequences
Nature (2023)
-
Olfactory navigation in arthropods
Journal of Comparative Physiology A (2023)
-
Maintenance of mitochondrial integrity in midbrain dopaminergic neurons governed by a conserved developmental transcription factor
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.