Abstract
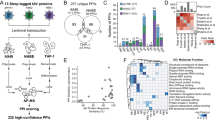
Viruses must enter host cells to replicate, assemble and propagate. Because of the restricted size of their genomes, viruses have had to evolve efficient ways of exploiting host cell processes to promote their own life cycles and also to escape host immune defence mechanisms1,2. Many viral open reading frames (viORFs) with immune-modulating functions essential for productive viral growth have been identified across a range of viral classes3,4. However, there has been no comprehensive study to identify the host factors with which these viORFs interact for a global perspective of viral perturbation strategies5,6,7,8,9,10,11. Here we show that different viral perturbation patterns of the host molecular defence network can be deduced from a mass-spectrometry-based host-factor survey in a defined human cellular system by using 70 innate immune-modulating viORFs from 30 viral species. The 579 host proteins targeted by the viORFs mapped to an unexpectedly large number of signalling pathways and cellular processes, suggesting yet unknown mechanisms of antiviral immunity. We further experimentally verified the targets heterogeneous nuclear ribonucleoprotein U, phosphatidylinositol-3-OH kinase, the WNK (with-no-lysine) kinase family and USP19 (ubiquitin-specific peptidase 19) as vulnerable nodes in the host cellular defence system. Evaluation of the impact of viral immune modulators on the host molecular network revealed perturbation strategies used by individual viruses and by viral classes. Our data are also valuable for the design of broad and specific antiviral therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Accessions
ArrayExpress
Data deposits
The protein interactions from this publication have been submitted to the IMEx consortium (http://imex.sf.net) through IntAct (identifier IM-17331). Mass spectrometry data are available at http://inhibitomev1.sf.net; microarray data were deposited in ArrayExpress (accession number E-MTAB-1148).
References
Watanabe, T., Watanabe, S. & Kawaoka, Y. Cellular networks involved in the influenza virus life cycle. Cell Host Microbe 7, 427–439 (2010)
Goff, S. P. Host factors exploited by retroviruses. Nature Rev. Microbiol. 5, 253–263 (2007)
Finlay, B. B. & McFadden, G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124, 767–782 (2006)
Bowie, A. G. & Unterholzner, L. Viral evasion and subversion of pattern-recognition receptor signalling. Nature Rev. 8, 911–922 (2008)
Jager, S. et al. Global landscape of HIV–human protein complexes. Nature 481, 365–370 (2012)
Shapira, S. D. et al. A physical and regulatory map of host–influenza interactions reveals pathways in H1N1 infection. Cell 139, 1255–1267 (2009)
Uetz, P. et al. Herpesviral protein networks and their interaction with the human proteome. Science 311, 239–242 (2006)
Krishnan, M. N. et al. RNA interference screen for human genes associated with West Nile virus infection. Nature 455, 242–245 (2008)
Brass, A. L. et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science 319, 921–926 (2008)
Konig, R. et al. Human host factors required for influenza virus replication. Nature 463, 813–817 (2010)
Karlas, A. et al. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature 463, 818–822 (2010)
Pichlmair, A. et al. IFIT1 is an antiviral protein that recognizes 5′-triphosphate RNA. Nature Immunol. 12, 624–630 (2011)
Li, S., Wang, L., Berman, M., Kong, Y. Y. & Dorf, M. E. Mapping a dynamic innate immunity protein interaction network regulating type I interferon production. Immunity 35, 426–440 (2011)
Cravatt, B. F., Simon, G. M. & Yates, J. R., III The biological impact of mass-spectrometry-based proteomics. Nature 450, 991–1000 (2007)
Randall, R. E. & Goodbourn, S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89, 1–47 (2008)
Schmolke, M. & Garcia-Sastre, A. Evasion of innate and adaptive immune responses by influenza A virus. Cell. Microbiol. 12, 873–880 (2010)
Rodriguez, J. J., Wang, L. F. & Horvath, C. M. Hendra virus V protein inhibits interferon signaling by preventing STAT1 and STAT2 nuclear accumulation. J. Virol. 77, 11842–11845 (2003)
Glatter, T., Wepf, A., Aebersold, R. & Gstaiger, M. An integrated workflow for charting the human interaction proteome: insights into the PP2A system. Mol. Syst. Biol. 5, 237 (2009)
Lynn, D. J. et al. InnateDB: facilitating systems-level analyses of the mammalian innate immune response. Mol. Syst. Biol. 4, 218 (2008)
Burkard, T. R. et al. Initial characterization of the human central proteome. BMC Syst. Biol. 5, 17 (2011)
Albert, R., Jeong, H. & Barabasi, A. L. Error and attack tolerance of complex networks. Nature 406, 378–382 (2000)
Hassink, G. C. et al. The ER-resident ubiquitin-specific protease 19 participates in the UPR and rescues ERAD substrates. EMBO Rep. 10, 755–761 (2009)
Arima, N. et al. Modulation of cell growth by the hepatitis C virus nonstructural protein NS5A. J. Biol. Chem. 276, 12675–12684 (2001)
Lu, Y. et al. USP19 deubiquitinating enzyme supports cell proliferation by stabilizing KPC1, a ubiquitin ligase for p27Kip1. Mol. Cell. Biol. 29, 547–558 (2009)
Valente, S. T. & Goff, S. P. Inhibition of HIV-1 gene expression by a fragment of hnRNP U. Mol. Cell 23, 597–605 (2006)
Hasegawa, Y. et al. The matrix protein hnRNP U is required for chromosomal localization of Xist RNA. Dev. Cell 19, 469–476 (2010)
Schroder, M., Baran, M. & Bowie, A. G. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. EMBO J. 27, 2147–2157 (2008)
Moniz, S. & Jordan, P. Emerging roles for WNK kinases in cancer. Cell. Mol. Life Sci. 67, 1265–1276 (2010)
Zybailov, B. et al. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J. Proteome Res. 5, 2339–2347 (2006)
Appel, N. et al. Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly. PLoS Pathog. 4, e1000035 (2008)
Pietschmann, T. et al. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc. Natl Acad. Sci. USA 103, 7408–7413 (2006)
Reiss, S. et al. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe 9, 32–45 (2011)
Habjan, M., Penski, N., Spiegel, M. & Weber, F. T7 RNA polymerase-dependent and -independent systems for cDNA-based rescue of Rift Valley fever virus. J. Gen. Virol. 89, 2157–2166 (2008)
Dittmann, J. et al. Influenza A virus strains differ in sensitivity to the antiviral action of Mx-GTPase. J. Virol. 82, 3624–3631 (2008)
Keating, S. E., Maloney, G. M., Moran, E. M. & Bowie, A. G. IRAK-2 participates in multiple Toll-like receptor signaling pathways to NFκB via activation of TRAF6 ubiquitination. J. Biol. Chem. 282, 33435–33443 (2007)
Haura, E. B. et al. Using iTRAQ combined with tandem affinity purification to enhance low-abundance proteins associated with somatically mutated EGFR core complexes in lung cancer. J. Proteome Res. 10, 182–190 (2010)
Burckstummer, T. et al. An efficient tandem affinity purification procedure for interaction proteomics in mammalian cells. Nature Methods 3, 1013–1019 (2006)
Kislinger, T. et al. Global survey of organ and organelle protein expression in mouse: combined proteomic and transcriptomic profiling. Cell 125, 173–186 (2006)
Schaefer, C. F. et al. PID: the Pathway Interaction Database. Nucleic Acids Res. 37, 674–679 (2009)
Acknowledgements
We thank C. Basler, A. Bergthaler, K.-K. Conzelmann, A. Garcia-Sastre, M. Hardy, W. Kaiser, E. Mühlberger, R. Randall, B. Roizman, N. Ruggli, B. Sherry and T. Wolf for providing viral ORF cDNAs; P. Jordan for providing WNK expression constructs; S. Nakagawa for Flag-hnRNP-U; M. Sophie-Hiet for GFP-NS5A; M. Zayas for the pFK-Jc1-NS5A-HA expression plasmid; E. Rudashevskaya, A. Stukalov, F. Breitwieser and M. Trippler for support; H. Pickersgill and T. Brummelkamp for critically reading the manuscript; C. Baumann for discussions; and M. Vidal for discussions and for sharing unpublished information. The work was funded by the Austrian Academy of Sciences, an i-FIVE European Research Council grant to G.S.-F., a European Molecular Biology Organization long-term fellowship to A.P. (ATLF 463-2008), Science Foundation Ireland grant 07/IN1/B934 to O.M. and A.G.B., Deutsche Forschungsgemeinschaft grants We 2616/5-2 and SFB 593/B13 to F.W., Ko1579/5-1 to G.K., and FOR1202, TP1 to R.B., and the German Ministry for Education and Research (Suszeptibilität bei Infektionen: HCV; TP1, 01KI 0786) to R.B. J.C. is funded by the Austrian Ministry of Science and Research (GEN-AU/BIN).
Author information
Authors and Affiliations
Contributions
A.P., G.A., O.M., R.S., M.H., M.B., A.S., C.A.E., A.G., A.C.M., A.F., C.H., S.G., F.W. and G.K. performed experiments. A.P. and G.S.-F. conceived the study. A.P., G.A., R.B., A.G.B. and G.S.-F. designed experiments. K.K., A.C.M., K.L.B. and J.C. performed mass spectrometry and bioinformatic data analysis. T.B., K.L., S.G., G.K., F.W., R.B. and A.G.B. provided critical material. All authors contributed to the discussion of results and participated in manuscript preparation. A.P., K.K., J.C. and G.S.-F. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-15. (PDF 2380 kb)
Supplementary Tables
This zipped file contains Supplementary Tables 1-9 (ZIP 766 kb)
Rights and permissions
About this article
Cite this article
Pichlmair, A., Kandasamy, K., Alvisi, G. et al. Viral immune modulators perturb the human molecular network by common and unique strategies. Nature 487, 486–490 (2012). https://doi.org/10.1038/nature11289
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11289
This article is cited by
-
Prediction of motif-mediated viral mimicry through the integration of host–pathogen interactions
Archives of Microbiology (2024)
-
A binary interaction map between turnip mosaic virus and Arabidopsis thaliana proteomes
Communications Biology (2023)
-
Comparative genomics and integrated system biology approach unveiled undirected phylogeny patterns, mutational hotspots, functional patterns, and molecule repurposing for monkeypox virus
Functional & Integrative Genomics (2023)
-
Cell-based receptor discovery identifies host factors specifically targeted by the SARS CoV-2 spike
Communications Biology (2022)
-
Nipah virus W protein harnesses nuclear 14-3-3 to inhibit NF-κB-induced proinflammatory response
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.