Abstract
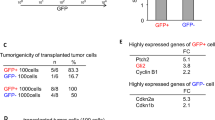
Glioblastoma multiforme is the most common primary malignant brain tumour, with a median survival of about one year1. This poor prognosis is due to therapeutic resistance and tumour recurrence after surgical removal. Precisely how recurrence occurs is unknown. Using a genetically engineered mouse model of glioma, here we identify a subset of endogenous tumour cells that are the source of new tumour cells after the drug temozolomide (TMZ) is administered to transiently arrest tumour growth. A nestin-ΔTK-IRES-GFP (Nes-ΔTK-GFP) transgene that labels quiescent subventricular zone adult neural stem cells also labels a subset of endogenous glioma tumour cells. On arrest of tumour cell proliferation with TMZ, pulse-chase experiments demonstrate a tumour re-growth cell hierarchy originating with the Nes-ΔTK-GFP transgene subpopulation. Ablation of the GFP+ cells with chronic ganciclovir administration significantly arrested tumour growth, and combined TMZ and ganciclovir treatment impeded tumour development. Thus, a relatively quiescent subset of endogenous glioma cells, with properties similar to those proposed for cancer stem cells, is responsible for sustaining long-term tumour growth through the production of transient populations of highly proliferative cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chen, J., McKay, R. M. & Parada, L. F. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell 149, 36–47 (2012)
Alcantara Llaguno, S. et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 15, 45–56 (2009)
Kwon, C. H. et al. Pten haploinsufficiency accelerates formation of high grade astrocytomas. Cancer Res. 68, 3286–3294 (2008)
Zhu, Y. et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell 8, 119–130 (2005)
Yu, T. S. et al. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J. Neurosci. 28, 12901–12912 (2008)
Ishii-Morita, H. et al. Mechanism of ‘bystander effect’ killing in the herpes simplex thymidine kinase gene therapy model of cancer treatment. Gene Ther. 4, 244–251 (1997)
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005)
Garcia, A. D. et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nature Neurosci. 7, 1233–1241 (2004)
Deng, W. et al. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 29, 13532–13542 (2009)
Singer, B. H. et al. Compensatory network changes in the dentate gyrus restore long-term potentiation following ablation of neurogenesis in young-adult mice. Proc. Natl Acad. Sci. USA 108, 5437–5442 (2011)
Snyder, J. S. et al. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461 (2011)
Bao, S. et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 66, 7843–7848 (2006)
Liu, C. et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell 146, 209–221 (2011)
Clarke, M. F. et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 66, 9339–9344 (2006)
Boiko, A. D. et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 466, 133–137 (2010)
Ishizawa, K. et al. Tumor-initiating cells are rare in many human tumors. Cell Stem Cell 7, 279–282 (2010)
Kelly, P. N. et al. Tumor growth need not be driven by rare cancer stem cells. Science 317, 337 (2007)
Quintana, E. et al. Efficient tumour formation by single human melanoma cells. Nature 456, 593–598 (2008)
Vega, C. J. & Peterson, D. A. Stem cell proliferative history in tissue revealed by temporal halogenated thymidine analog discrimination. Nature Methods 2, 167–169 (2005)
Acknowledgements
The authors thank S. McKinnon, A. Deshaw, L. McClellan, S. Kennedy and P. Leake for technical assistance, and Parada laboratory members for helpful suggestions and discussion. CldU and IdU preparation and staining protocol was provided by D. A. Peterson at Rosalind Franklin University. This work was supported by grants awarded to S.G.K. (RO1 NS048192-01) and to L.F.P. by the Goldhirsh Foundation, the James S. McDonnell Foundation (JSMF-220020206), Cancer Prevention Research Institute of Texas (RP 100782) and the National Institutes of Health (R01 CA131313). L.F.P. is an American Cancer Society Research Professor.
Author information
Authors and Affiliations
Contributions
J.C. and Y.L. performed the experiments. T.-S.Y. and S.G.K. contributed vital reagents. J.C. and L.F.P. designed the experiments. J.C., R.M.M., D.K.B. and L.F.P. analysed the data. J.C., R.M.M. and L.F.P. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-7. (PDF 18337 kb)
Rights and permissions
About this article
Cite this article
Chen, J., Li, Y., Yu, TS. et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488, 522–526 (2012). https://doi.org/10.1038/nature11287
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11287
This article is cited by
-
Threonine fuels glioblastoma through YRDC-mediated codon-biased translational reprogramming
Nature Cancer (2024)
-
Chemotactic nanomotor for multimodal combined therapy of glioblastoma
Science China Chemistry (2024)
-
BMP signaling in cancer stemness and differentiation
Cell Regeneration (2023)
-
Pancreatic cancer stemness: dynamic status in malignant progression
Journal of Experimental & Clinical Cancer Research (2023)
-
CRISPR-Cas9 knockout screen identifies novel treatment targets in childhood high-grade glioma
Clinical Epigenetics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.