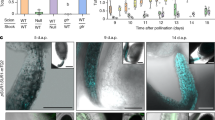
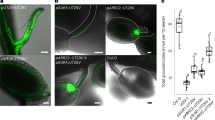
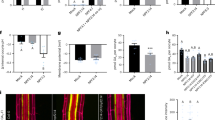
Abstract
In plants, transport processes are important for the reallocation of defence compounds to protect tissues of high value1, as demonstrated in the plant model Arabidopsis, in which the major defence compounds, glucosinolates2, are translocated to seeds on maturation3. The molecular basis for long-distance transport of glucosinolates and other defence compounds, however, remains unknown. Here we identify and characterize two members of the nitrate/peptide transporter family, GTR1 and GTR2, as high-affinity, proton-dependent glucosinolate-specific transporters. The gtr1 gtr2 double mutant did not accumulate glucosinolates in seeds and had more than tenfold over-accumulation in source tissues such as leaves and silique walls, indicating that both plasma membrane-localized transporters are essential for long-distance transport of glucosinolates. We propose that GTR1 and GTR2 control the loading of glucosinolates from the apoplasm into the phloem. Identification of the glucosinolate transporters has agricultural potential as a means to control allocation of defence compounds in a tissue-specific manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Feeny, P. in Biochemical Interaction Between Plants and Insects (eds Wallace, J. W. & Mansel, R. L. ) 1–40 (Plenum, 1976)
Halkier, B. A. & Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 57, 303–333 (2006)
Nour-Eldin, H. & Halkier, B. Piecing together the transport pathway of aliphatic glucosinolates. Phyochemistry Rev. 8, 53–67 (2009)
McKey, D. Adaptive patterns in alkaloid physiology. Am. Nat. 108, 305–320 (1974)
Züst, T., Joseph, B., Shimizu, K. K., Kliebenstein, D. J. & Turnbull, L. A. Using knockout mutants to reveal the growth costs of defensive traits. Proc. Biol. Sci. 278, 2598–2603 (2011)
Ohnmeiss, T. E. & Baldwin, I. T. Optimal defense theory predicts the ontogeny of an induced nicotine defense. Ecology 81, 1765–1783 (2000)
Wink, M. Wounding-induced increase of quinolizidine alkaloid accumulation in lupin leaves. Z. Naturforschung 38, 905–909 (1983)
Brown, P. D., Tokuhisa, J. G., Reichelt, M. & Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62, 471–481 (2003)
Townsend, B. J. & Llewellyn, D. J. Reduced terpene levels in cottonseed add food to fiber. Trends Biotechnol. 25, 239–241 (2007)
Mailer, R., McFadden, A., Ayton, J. & Redden, B. Anti-nutritional components, fibre, sinapine and glucosinolate content, in Australian canola (Brassica napus L.) meal. J. Am. Oil Chem. Soc. 85, 937–944 (2008)
Wu, S. & Chappell, J. Metabolic engineering of natural products in plants; tools of the trade and challenges for the future. Curr. Opin. Biotechnol. 19, 145–152 (2008)
Enneking, D. & Wink, M. in Proceedings of the Third International Food Legumes Research Conference, Linking Research and Marketing Opportunities for Pulses in the 21st Century (ed. Knight, R. ) 671–683 (Kluwer, 2000)
Chen, S., Petersen, B. L., Olsen, C. E., Schulz, A. & Halkier, B. A. Long-distance phloem transport of glucosinolates in Arabidopsis. Plant Physiol. 127, 194–201 (2001)
Ellerbrock, B. L., Kim, J. H. & Jander, G. Contribution of glucosinolate transport to Arabidopsis defense responses. Plant Signal. Behav. 2, 282–283 (2007)
Nour-Eldin, H. H., Norholm, M. H. & Halkier, B. A. Screening for plant transporter function by expressing a normalized Arabidopsis full-length cDNA library in Xenopus oocytes. Plant Methods 2, 17–25 (2006)
Wang, Y. Y. & Tsay, Y. F. Arabidopsis nitrate transporter NRT1.9 is important in phloem nitrate transport. Plant Cell 23, 1945–1957 (2011)
Fan, S. C., Lin, C. S., Hsu, P. K., Lin, S. H. & Tsay, Y. F. The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. Plant Cell 21, 2750–2761 (2009)
Bednarek, P. et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323, 101–106 (2009)
Koroleva, O. A. et al. Identification of a new glucosinolate-rich cell type in Arabidopsis flower stalk. Plant Physiol. 124, 599–608 (2000)
Balbi, V. & Devoto, A. Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol. 177, 301–318 (2008)
Foster, R., Williamson, C. S. & Lunn, J. Culinary oils and their health effects. Nutrition Bulletin 34, 4–47 (2009)
Snowdon, R., Luhs, W. & Friedt, W. in Genome Mapping and Molecular Breeding in Plants (ed. Kole, C. ) 55–114 (Springer, 2007)
Wittkop, B., Snowdon, R. & Friedt, W. Status and perspectives of breeding for enhanced yield and quality of oilseed crops for Europe. Euphytica 170, 131–140 (2009)
Clarke, D. B. Glucosinolates, structures and analysis in food. Anal. Methods 2, 310–325 (2010)
Zhang, J. et al. Metabolite profiling of Arabidopsis seedlings in response to exogenous sinalbin and sulfur deficiency. Phytochemistry 72, 1767–1778 (2011)
Gottwald, J. R., Krysan, P. J., Young, J. C., Evert, R. F. & Sussman, M. R. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc. Natl Acad. Sci. USA 97, 13979–13984 (2000)
Vert, G. et al. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14, 1223–1233 (2002)
Nour-Eldin, H. H., Hansen, B. G., Norholm, M. H. H., Jensen, J. K. & Halkier, B. A. Advancing uracil-excision based cloning towards an ideal technique for cloning PCR fragments. Nucleic Acids Res. 34, e122 (2006)
Geu-Flores, F., Nour-Eldin, H. H., Nielsen, M. T. & Halkier, B. A. USER fusion: a rapid and efficient method for simultaneous fusion and cloning of multiple PCR products. Nucleic Acids Res. 35, e55 (2007)
Nørholm, M. A mutant Pfu DNA polymerase designed for advanced uracil-excision DNA engineering. BMC Biotechnol. 10, 21–27 (2010)
Acknowledgements
We thank M. Palmgren, A. Schulz and D. Kliebenstein for comments on the manuscript and H. K. S. Jepsen, L. B. Møller and A. Kraus for technical assistance. We thank D. Klærke and technicians T. K. Soland, B. L. Christensen, M. Olsen and C. Derrer for providing X. laevis oocytes. We thank the Salk, Stanford, Plant Gene Expression Center (SSP) Consortium and the RIKEN Genome Science Center for providing full-length cDNAs. Imaging data were achieved at the Center for Advanced Bioimaging, University of Copenhagen. H.H.N-.E. was supported by the Danish Research Council for Technology and Production (FTP) grant 09-065827/274-08-0354. M.B. was supported by a Marie Curie fellowship (grant PIEF-GA-2008-221236). I.D. was supported by a Marie-Curie Career Integration Grant of the European Union (FP7-PEOPLE-2011-CIG No. 303674 (Regopoc)). D.G. was supported by the Deutsche Forschungsgemeinschaft grant GE2195/1-1 and R.H. by Deutsche Forschungsgemeinschaft grants within FOR 1061. B.A.H. is partner of The VKR Research Centre for Pro-Active Plants funded by the Villum Kann Rasmussen Foundation. Funding for DynaMo Center of Excellence is provided by the Danish National Research Foundation.
Author information
Authors and Affiliations
Contributions
H.H.N-.E. identified the GTRs, designed the study, performed the phylogenetic analyses, contributed to biochemical characterization, data analysis, confocal localization experiments and analyses of metabolite profiling data and wrote the paper. T.G.A. performed the confocal localization experiments, generated and characterized glucosinolate in the gtr knockout mutants and contributed to biochemical characterization, study design and preparation of the manuscript. M.B. purified glucosinolates necessary for biochemical characterization and contributed to study design, data analysis and preparation of the manuscript. S.R.M. generated and analysed GTR-promoter-β-glucuronidase lines, contributed to confocal localization experiments and performed germination experiments. M.E.J. performed metabolite profiling of seeds and analysed data and performed seed morphology and yield analyses. C.E.O. performed LC–MS analyses for the transporter library screen and for metabolite profiling. I.D. performed theoretical discussion on GTR kinetics. R.H. contributed to the study design and data analyses. D.G. performed the biophysical characterization of the GTR transport mechanism and contributed to the theoretical discussion on GTR kinetics, study design and preparation of the manuscript. B.A.H. contributed to and supervised the study design and contributed to data analyses and preparation of the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The subject matter of this publication forms the basis of a patent application USSN 61/362,390 and EP10075299.7.
Supplementary information
Supplementary Information 1
This file contains Supplementary Figures 1, 3-7 and legend for Supplementary Figure 2 (see separate file for Supplementary Figure 2), additional references, Supplementary Materials and Methods, Supplementary References, Supplementary Table 1, a Supplementary Discussion and full legends for Supplementary Movies 1-4. (PDF 1294 kb)
Supplementary Information 2
This file contains Supplementary Figure 2 (see Supplementary Information file for legend). (PDF 2312 kb)
Supplementary Movie 1
Representative movie of z-stack spanning 27.0 μm of five-week-old leaves from stable Arabidopsis lines transformed with pGTR1(2kb)-GTR1(genomic fragment)-YFP(venus)-GTR1_3’UTR(0.3kb) (seven independent lines), showing plasma membrane localization of GTR1. (MOV 1303 kb)
Supplementary Movie 2
This movie shows background YFP fluorescence in non-transformed plants. (MOV 737 kb)
Supplementary Movie 3
Representative movie of a z-stack spanning 16.4 μm of five-week-old leaves from stable Arabidopsis lines transformed with pGTR2(2kb)-GTR2 (genomic fragment)-mOrange-GTR2_3'UTR(0.3kb) (22 independent lines), showing plasma membrane localization of GTR2. (MOV 862 kb)
Supplementary Movie 4
This movie shows background mOrange fluorescence in non-transformed plants. (MOV 275 kb)
Rights and permissions
About this article
Cite this article
Nour-Eldin, H., Andersen, T., Burow, M. et al. NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature 488, 531–534 (2012). https://doi.org/10.1038/nature11285
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11285
This article is cited by
-
An UMAMIT-GTR transporter cascade controls glucosinolate seed loading in Arabidopsis
Nature Plants (2024)
-
Double haploid production using microspore culture is a useful breeding method in the modulation of glucosinolates contents in Radish (Raphanus sativus L.)
Horticulture, Environment, and Biotechnology (2024)
-
Export of defensive glucosinolates is key for their accumulation in seeds
Nature (2023)
-
Comparative responses of sulforaphene contents between radish (Raphanus sativus L.) and Baemuchae (xBrassicoraphanus) during seed development
Horticulture, Environment, and Biotechnology (2023)
-
Gibberellin and abscisic acid transporters facilitate endodermal suberin formation in Arabidopsis
Nature Plants (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.