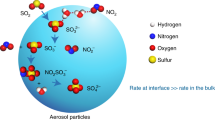
Abstract
Atmospheric oxidation is a key phenomenon that connects atmospheric chemistry with globally challenging environmental issues, such as climate change1, stratospheric ozone loss2, acidification of soils and water3, and health effects of air quality4. Ozone, the hydroxyl radical and the nitrate radical are generally considered to be the dominant oxidants that initiate the removal of trace gases, including pollutants, from the atmosphere. Here we present atmospheric observations from a boreal forest region in Finland, supported by laboratory experiments and theoretical considerations, that allow us to identify another compound, probably a stabilized Criegee intermediate (a carbonyl oxide with two free-radical sites) or its derivative, which has a significant capacity to oxidize sulphur dioxide and potentially other trace gases. This compound probably enhances the reactivity of the atmosphere, particularly with regard to the production of sulphuric acid, and consequently atmospheric aerosol formation. Our findings suggest that this new atmospherically relevant oxidation route is important relative to oxidation by the hydroxyl radical, at least at moderate concentrations of that radical. We also find that the oxidation chemistry of this compound seems to be tightly linked to the presence of alkenes of biogenic origin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Liao, H. et al. Effect of chemistry-aerosol-climate coupling on predictions of future climate and future levels of tropospheric ozone and aerosols. J. Geophys. Res.. 114, D10306, http://dx.doi.org/10.1029/2008JD010984 (2009)
Rowland, F. S. Stratospheric ozone depletion. Phil. Trans. R. Soc. Lond. B 361, 769–790 (2006)
Likens, G. E., Bormann, F. H. & Johnson, N. M. Acid rain. Environment 14, 33–40 (1974)
Fenger, J. Air pollution in the last 50 years — from local to global. Atmos. Environ. 43, 13–22 (2009)
Lelieveld, J. et al. Atmospheric oxidation capacity sustained by a tropical forest. Nature 452, 737–740 (2008)
Arneth, A. et al. Terrestrial biogeochemical feedbacks in the climate system. Nature Geosci. 3, 525–532 (2010)
Su, H. et al. Soil nitrite as a source of atmospheric HONO and OH radicals. Science 333, 1616–1618 (2011)
Di Carlo, P. et al. Missing OH reactivity in a forest: evidence for unknown reactive biogenic VOCs. Science 304, 722–725 (2004)
Lou, S. et al. Atmospheric OH reactivities in the Pearl River Delta – China in summer 2006: measurements and model results. Atmos. Chem. Phys. 10, 11243–11260 (2010)
Hofzumahaus, A. et al. Amplified trace gas removal in the troposphere. Science 324, 1702–1704 (2009)
Montzka, S. A. et al. Small interannual variability of global atmospheric hydroxyl. Science 331, 67–70 (2011)
Sipilä, M. et al. The role of sulfuric acid in atmospheric nucleation. Science 327, 1243–1246 (2010)
Eisele, F. L. & Tanner, D. J. Ion-assisted tropospheric OH measurements. J. Geophys. Res. 96, 9295–9308 (1991)
Petäjä, T. et al. Sulfuric acid and OH concentrations in a boreal forest site. Atmos. Chem. Phys. 9, 7435–7448 (2009)
Hakola, H. Seasonal variation of VOC concentrations above a boreal coniferous forest. Atmos. Environ. 37, 1623–1634 (2003)
Lappalainen, H. K. et al. Day-time concentrations of biogenic volatile organic compounds in a boreal forest canopy and their relation to environmental and biological factors. Atmos. Chem. Phys. 9, 5447–5459 (2009)
Cocks, A. T., Fernando, R. P. & Fletcher, I. S. The gas-phase reaction of the methylperoxy radical with sulphur dioxide. Atmos. Environ. 20, 2359–2366 (1986)
Xie, Z. D. Formation mechanism of condensation nuclei in nighttime atmosphere and the kinetics of the SO2-O3-NO2 system. J. Phys. Chem. 96, 1543–1547 (1992)
Kurtén, T., Lane, J. R., Jørgensen, S. & Kjaergaard, H. Nitrate radical addition-elimination reactions of atmospherically relevant sulfur-containing molecules. Phys. Chem. Chem. Phys. 12, 12833–12839 (2010)
Kurtén, T., Lane, J. R., Jørgensen, S. & Kjaergaard, H. A. Computational study of the oxidation of SO2 to SO3 by gas-phase organic oxidant. J. Phys. Chem. A 115, 8669–8681 (2011)
Hatakeyama, S., Kobayashi, H., Lin, Z.-Y., Tagaki, H. & Akimoto, H. Mechanism for the reaction of H2COO with SO2 . J. Phys. Chem. 90, 4131–4135 (1986)
Johnson, D., Lewin, A. G. & Marston, G. The effect of Criegee-intermediate scavengers on the OH yield from the reaction of ozone with 2-methylbut-2-ene. J. Phys. Chem. A 105, 2933–2935 (2001)
Jiang, L., Xu, Y. & Ding, A. J. Reaction of stabilized Criegee intermediates from ozonolysis of limonene with sulfur dioxide: ab initio and DFT study. J. Phys. Chem. A 114, 12452–1246 (2010)
Welz, O. et al. Direct kinetic measurements of Criegee intermediate (CH2OO) formed by reaction of CH2I with O2 . Science 335, 204–207 (2012)
Cox, R. A. & Penkett, S. A. Oxidation of atmospheric SO2 by products of the ozone-olefin reaction. Nature 230, 321–322 (1971)
Drozd, G. T., Kroll, J. & Donahue, N. M. 2,2-Dimethyl-2-butene (TME) ozonolysis: pressure dependence of stabilized Criegee intermediates and evidence of stabilized vinyl hydroperoxides. J. Phys. Chem. A 115, 161–166 (2011)
Heald, C. L. et al. Predicted change in global secondary organic aerosol concentrations in response to future climate, emissions, and land use change. J. Geophys. Res.. 113, D05211, http://dx.doi.org/10.1029/2007JD009092 (2008)
Spracklen, D. V. et al. Contribution of particle formation to global cloud condensation nuclei concentrations. Geophys. Res. Lett.. 35, L06808, http://dx.doi.org/10.1029/2007GL033038 (2008)
Arneth, A., Unger, N., Kulmala, M. & Andreae, M. O. Clean the air, heat the planet? Science 326, 672–673 (2009)
DeMore, W. et al. Chemical Kinetics and Photochemical Data for Use in Stratospheric Modeling. Evaluation 12. JPL Publication 97-4 (Jet Propulsion Laboratory, 1997)
Tanner, D. J., Jefferson, A. & Eisele, F. L. Selected ion chemical ionization mass spectrometric measurement of OH. J. Geophys. Res. 102, 6415–6425 (1997)
Mauldin, R. L., III et al. OH measurements during ACE-1: observations and model comparisons. J. Geophys. Res. 103, 16713–16729 (1998)
Berndt, T. et al. Laboratory study on new particle formation from the reaction OH + SO2: influence of experimental conditions, H2O vapour, NH3 and the amine tert-butylamine on the overall process. Atmos. Chem. Phys. 10, 7101–7116 (2010)
Hari, P. & Kulmala, M. Station for measuring ecosystem atmosphere relations (SMEAR II). Boreal Environ. Res. 10, 315–322 (2005)
Acknowledgements
We thank K. Pielok and A. Rohmer for technical assistance. This work was partially funded by the European Commission Sixth Framework programme project EUCAARI, contract no. 036833-2 (EUCAARI), the Academy of Finland (251427, 139656, Finnish centre of excellence 141135), the European Research Council (ATMNUCLE), the Kone Foundation, the Väisälä Foundation, the Maj and Tor Nessling Foundation (2010212), the Otto Malm Foundation and the US National Science Foundation.
Author information
Authors and Affiliations
Contributions
R.L.M., T.B. and M.S. designed the experiments, R.L.M., T.B., M.S. and S.K. performed the laboratory experiments, R.L.M., T.P. and M.S. conducted the field measurements, T.B., T.K., and P.P. performed the model and theoretical calculations, and R.L.M., T.B., M.S. and P.P. analysed the data. All authors (R.L.M., T.B., M.S., P.P., T.P., S.K., T.K., F.S., V.-M.K., and M.K.) contributed to the interpretation and to manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, additional references and Supplementary Figures 1-6. (PDF 598 kb)
Rights and permissions
About this article
Cite this article
Mauldin III, R., Berndt, T., Sipilä, M. et al. A new atmospherically relevant oxidant of sulphur dioxide. Nature 488, 193–196 (2012). https://doi.org/10.1038/nature11278
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11278
This article is cited by
-
Fundamentals and catalytic applications of single-atom alloys
Science China Materials (2024)
-
Direct sulfuric acid formation from the gas-phase oxidation of reduced-sulfur compounds
Nature Communications (2023)
-
Photodissociation pathways in the simplest Criegee intermediate: a semi-classical investigation
Journal of Chemical Sciences (2023)
-
New particle formation from agricultural recycling of organic waste products
npj Climate and Atmospheric Science (2021)
-
Surprisingly long lifetime of methacrolein oxide, an isoprene derived Criegee intermediate, under humid conditions
Communications Chemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.